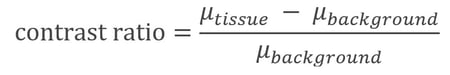X-ray computed tomography is an X-ray absorption imaging technique for which X-ray absorption scales with increased atomic number. Because organic materials are mostly composed of light elements, like carbon, nitrogen, and oxygen, these samples absorb fewer X-rays. As a result, CT images of organic materials often suffer from low contrast. To improve contrast, we stain organic materials with solutions containing elements with higher density, such as iodine and tungsten. These ‘heavy’ elements penetrate and bind to organic material resulting in CT images with higher contrast, as shown in the example below.
2. How to prepare samples before staining
Prior to staining, samples require preservation to prevent decay due to autolysis or putrefaction. This process, called fixation, terminates ongoing biochemical reactions and often increases tissue’s mechanical strength and stability. Most importantly, fixation permanently preserves the tissue in a lifelike state. For microscopy, chemical agents are usually used as fixatives.
There are generally two types of chemical fixation: cross-linking and coagulation. Cross-linking fixatives causes covalent bonds to form within and between proteins. This results in tissue stiffening as well as resistance to degradation. Coagulation fixatives remove water from tissues leading to coagulation and denaturation of proteins. Typically, the sample is immersed in the fixative agent for a length of time, usually up to 48 hours or longer.
Popular fixing agents include solutions containing formaldehyde and alcohol. As described in Metscher, BMC Physiology,(2009) 9:11, typical fixatives include formalin, Bouin’s fluid, ethanol, and glutaraldehyde. As you might expect, fixation may cause tissues and cells to shrink, swell or deform. So, the best fixative for your sample will depend on the type of sample you have – for example, plant, animal, lipid – as well as the sample thickness. Don’t be afraid to experiment to find the best one for your samples. Here are some links to guide you in the preparation of and using fixatives.
3. What are typical stains used for organic samples?
Now that your sample is preserved, you’re ready to stain. And, for those using certain types of chemical agents like ethanolic phosphotungstic acid (ePTA), you can combine the fixation and staining steps. This is particularly useful for the preparation of plants for X-ray CT data collection, as shown in Duncan et al., Plant Physiology, (2021) 0:1-15. Alternatively, you can fix and then stain in an aqueous PTA solution. Important factors to consider when staining are your choice of stain, the staining time, stain concentration, and stain toxicity.
The point of staining is to introduce heavy elements into your sample that will cause greater X-ray absorption and thus improve X-ray contrast. Common staining agents include solutions containing iodine, tungsten, molybdenum, osmium, indium, and others. Each of these elements has an atomic number ranging from 42 to 76, which means they are far heavier than carbon, nitrogen, and oxygen with atomic numbers of 8 or less.
Below is a table of different stains compiled from various publications that have been found to give good X-ray contrast to a wide variety of tissues. These include phosphotungstic acid and iodine solutions which have been used widely for a number of years. Another group, polyoxometalates (POMs), have been characterized more recently as effective contrast agents(Kerckhofs et al., Biomaterials (2018) 159:1). POMs are inorganic molecular metal oxide clusters that contain transition metals (typically V, Mo, W). The common usage of each these staining agents is to increase contrast, however some are preferred because they are less prone to cause tissue shrinkage or because they do not alter immunoreactivity.
|
Abbreviation
|
Staining agent components
|
|
PTA
|
1% (w/v) phosphotungstic acid in water
|
|
ePTA
|
1% PTA (w/v) in 90% ethanol in water (v/v)
|
|
IKI (Lugol’s solution)
|
1% iodine metal (I2) + 2% potassium iodide (KI) in water
|
|
I2E, I2M
|
1% iodine metal (I2) dissolved in 100% ethanol (I2E) or methanol (I2M)
|
|
POMs
|
Mono-WD POM (35.0 mg/mL) with 3.0 mg/mL LiCl, dissolved in PBS
(de Bournonville, et al., Acta Biomaterialia (2020) 105:253)
|

Stain time will typically depend on the size of your sample, the type of stain, and your stain concentration. Generally, staining times can vary from 24 hours up to several months. Duncan et al. (2021, see previous citation) cites that staining for too short of a time gave inferior X-ray contrast results while staining longer did not adversely affect contrast. Stain uptake occurs via diffusion through tissue, so larger samples will take longer to stain. One group described their results and quantified stain penetration via a simple contrast ratio by looking at 16-bit greyscale values in CT images (Swart et al., Scientific Reports, (2016) 6:39380):
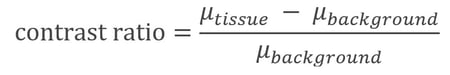
In this way, they evaluated stain uptake in Dipteran flies and provided some feedback for fixation and staining for these types of samples. Another recommended resource, included earlier in this article, describes sample preparation of economically and scientifically important plant systems for X-ray CT imaging (Duncan et al., Plant Physiology, (2021) 0:1-15). Many articles cite that they replenish stain solutions at some interval, usually 1 to 7 days.
Conclusion
In conclusion, the literature is rich with many examples and discussions on staining for X-ray imaging. Finding the best one for your sample will, of course, be an experiment, but the consensus seems to say that longer incubation times in staining solution are better with frequent replenishing along the way. In our own laboratory, I have had a lot of success using iodine and PTA containing solutions, so they are my default ‘go to’ choices. How about you? Do you have a favorite stain you use or a favorite resource recommendation? If so, please share it with us at info@rigaku.com.