Pharmaceutical Manufacturing
The globalization of the pharmaceutical supply chain and push towards 100% material inspection has led to a significant increase in the demand for cost effective, efficient and regulatory compliant methods for raw material identification and chemical verification. Strict quality assurance and quality control measures are more important than ever to ensuring the safety and efficacy of products before reaching consumers.
Traditional workflows involve sending samples to external laboratories for analysis. However, this can result in additional costs and time delays. By implementing a raw material identification that offers ease of use, a comprehensive range of sampling capabilities and rapid, customizable software at the point of need, a lean manufacturing process can be achieved.
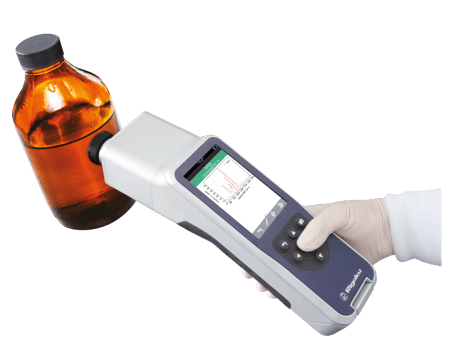
Pharmaceutical manufacturers can now achieve 100% raw material identification, verify chemicals and solvents, as well as authenticate final products quickly and confidently. Using Progeny 1064nm handheld Raman, users can achieve U.S. FDA Good Manufacturing Practices (GMP) and comply with U.S. FDA 21 CFR Part 11. Raman spectroscopy is now recognized by the U.S. Pharmacopoeia and the European Pharmacopeia for raw material identification.

Applications
Where is there a need for real-time material identification and verification?
What others are saying
-

Another thing I like about Progeny is the software as it relates to data integrity, ALCOA and those type of principals that you’re operating under a GMP environment.
Read moreDr. Joseph StoltzSenior Principal ScientistPfizer -

I came at this with very little analytical background…and I found the instrument really easy to use…
Read moreDr. Mark LevineSenior Staff ScientistSanofi Genzyme -

We use Progeny at every point in our manufacturing.
Read moreMichelle O'ConnorAssociate DirectorLyndra Therapeutics
Recommended Product
Progeny
Versatile, handheld spectrometer for raw material identification and verification.
Application notes
The following application notes are relevant to this industry.

Contact Us
Whether you're interested in getting a quote, want a demo, need technical support, or simply have a question, we're here to help.