Application Note B-XRD1117
Introduction
In order to develop new materials that have desired properties, it is essential to evaluate the materials under various atmospheric environments. The infrared heating high-temperature attachment Reactor X has a corrosion-resistant sample chamber separated from the heater section, so it can be used to perform high-temperature XRD measurements under various atmospheres, such as hydrogen, ammonia, high humidity and so forth. Using Reactor X with a 2D detector capable of high-speed XRD measurement, it is possible to investigate in detail rapid phase transitions under heating in various atmospheres.
Measurement and analysis
The redox reaction of a Cu powder sample was observed with Reactor X. The X-ray diffraction measurement (duration time: 1 min) was repeated while increasing the temperature at 50˚C/min from RT to 800˚C, at which point H₂ (4%) gas was introduced while keeping the temperature at 800˚C. Fig. 1 shows the obtained profiles. During the temperature increase from RT to 800˚C (from the lower to the middle part of Fig. 1 (left)), the diffraction peaks of Cu shifted to lower 2θ angles due to thermal expansion. Then the diffraction pattern changed to that of Cu₂O and CuO. After introducing H₂ gas (from the middle to the upper part of Figure 1 (left)), the diffraction pattern changed to Cu₂O and finally Cu. Figure 2 compares the transition of the mass fractions of Cu, Cu₂O and CuO. The chart indicates that the redox reaction of Cu occurred through states where Cu₂O coexisted.
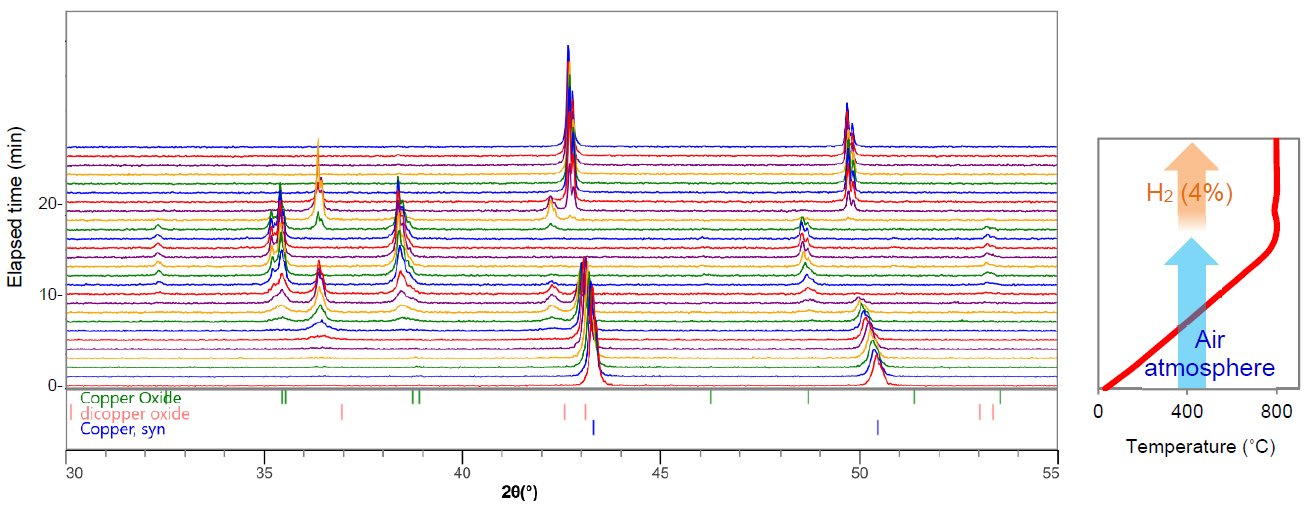
Figure 1: X-ray diffraction profiles (left) and the heating and atmosphere condition (right).
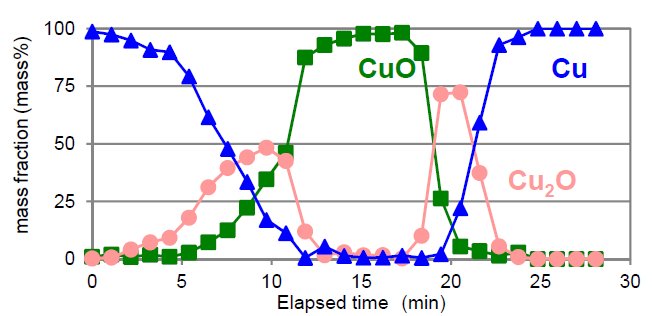
Figure 2: Result of quantitative analysis by Rietveld method.

