DSC
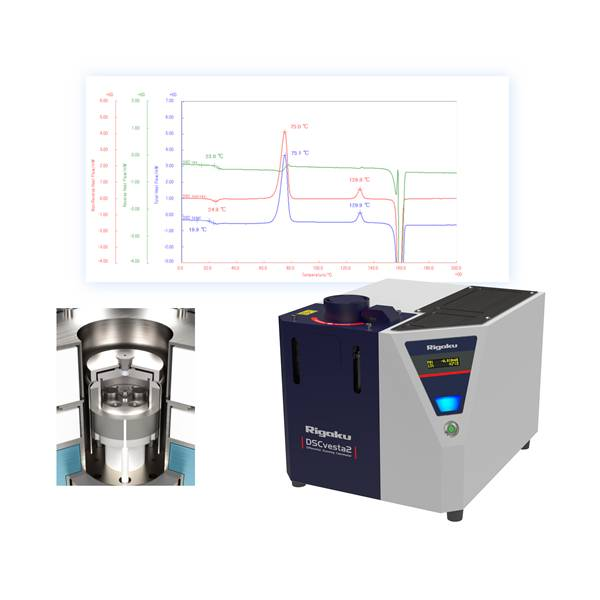
Differential Scanning Calorimetry (DSC) is an analytical technique used to measure how a material reacts to changes in temperature. By detecting tiny energy differences between a sample and a reference (often made of a-Al2O3), DSC reveals thermal events as the material is heated or cooled.
With DSC, you can evaluate key thermal properties such as:
- Phase transitions (melting, crystallization): DSC quantifies the heat absorbed or released when a material changes from solid to liquid, or vice versa—allowing precise determination of melting points and crystallization behavior.
- Glass transition temperature (Tg): Especially important in polymers, Tg marks the point where materials shift from a rigid, glassy state to a flexible, rubbery one.
- Chemical reactions and decomposition: DSC detects heat flow associated with chemical reactions, helping assess reaction kinetics and thermal stability.
- Specific heat capacity (Cp): DSC can also measure specific heat capacity, which is the amount of energy required to raise the temperature of a material.
DSC is widely used across industries such as materials science, pharmaceuticals, polymers, and food production. In pharmaceutical development, for example, DSC helps ensure the thermal stability of active ingredients. In plastics and polymer research, it provides essential data to optimize product performance.
With its ability to precisely measure heat flow, DSC plays a crucial role in product development and quality control, providing valuable insights into how materials behave thermally under real-world conditions.
Rigaku recommends the following products

Contact Us
Whether you're interested in getting a quote, want a demo, need technical support, or simply have a question, we're here to help.
