Application Note B-XRD1113
Introduction
The valence of metal ions in a crystal is an important structural parameter for understanding a material’s property and for designing new materials. There are numerous methods of experimentally determining the valence of a metal ion, including XAFS (X-ray Absorption Fine Structure), XPS (X-ray Photoelectron Spectroscopy), Mössbauer absorption spectroscopy, MEM (Maximum Entropy Method) and BVS (Bond Valence Sum). BVS is an empirical method based on Rietveld analysis that estimates the valence states based on the bond distances to neighboring atoms. Here, we illustrate the oxidation states of Mn oxides by BVS.
Measurements and results
X-ray powder diffraction of Mn₃O₄ and Mn₂O₃ were performed using Cu radiation. X‑ray fluorescence of Mn is removed by the XRF reduction mode of the detector. The d‑spacing is measured to be 0.82 Å (2θ/θ = 140˚), which corresponds to half to a third of the length of the Mn-O bond distance. BVS, which is computed from the Mn-O bond distances obtained from the Rietveld analysis, and the Bond Valence Parameters (Mn²⁺-O²⁻: 1.790 (3) and Mn³⁺-O²⁻: 1.760 (5)) (1), predicts the proper oxidation state of the Mn ion for a given crystal structure as shown in Fig.1. Table 1 and 2 show the BVS values for Mn ions in the Mn₃O₄ and Mn₂O₃ crystal structures, respectively. The reliability factors of the Rietveld analysis are Rwp = 4.49%、 Rp = 2.62%、S = 1.6093 for Mn₃O₄, and Rwp = 4.55%, Rp = 3.59%, S = 1.1949 for Mn₂O₃, respectively. BVS is useful for evaluating the stability and the validity of artificially designed crystal structures, in addition to being a simple approach for predicting and estimating the oxidation states of metal ions from the crystal structure.
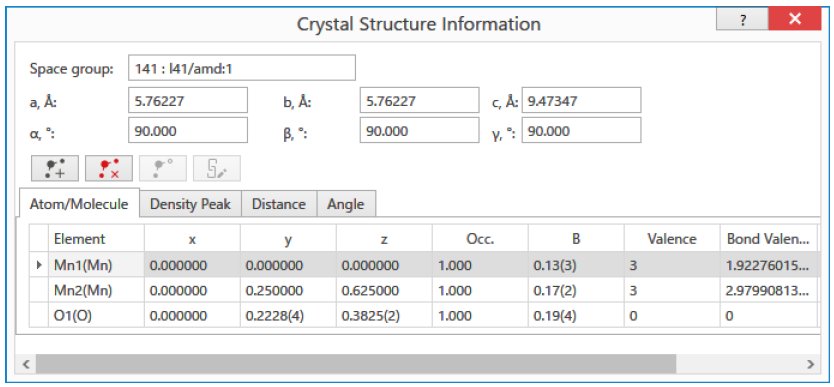
Figure 1: The input screen for the BVS calculation of Mn₃O₄. (When the Mn valences are input as trivalent, the BVS of Mn1 and Mn2 sites are calculated as 1.92 (divalent) and 2.98 (trivalent), respectively.)
Table 1: BVS of Mn ions and structural parameters of Mn₃O₄
| Space group | 141: I41/amd:1 | ||||
| a (Å) | 5.76227(10) | b (Å) | 5.76227(10) | c (Å) | 9.47347(18) |
| α (°) | 90.000 | β (°) | 90.000 | γ (°) | 90.000 |
| Element | x | y | z | Occupancy | B | BVS |
| Mn1 | 0.0000 | 0.0000 | 0.0000 | 1.0 | 0.13(3) | 2.085 |
| Mn2 | 0.0000 | 0.2500 | 0.6250 | 1.0 | 0.17(2) | 2.980 |
| O1 | 0.0000 | 0.2228(4) | 0.3825(2) | 1.0 | 0.19(4) | – |
Table 2: BVS of Mn ions and structural parameters of Mn₂O₃
| Space group | 206: Ia3 | ||||
| a (Å) | 9.41533(13) | b (Å) | 9.41533(13) | c (Å) | 9.41533(13) |
| α (°) | 90.000 | β (°) | 90.000 | γ (°) | 90.000 |
| Element | x | y | z | Occupancy | B | BVS |
| Mn1 | 0.0000 | 0.0000 | 0.0000 | 1.0 | 0.46(3) | 3.047 |
| Mn2 | 0.28519(6) | 0.0000 | 0.0000 | 1.0 | 0.50(3) | 3.026 |
| O1 | 0.1296(3) | 0.1473(3) | -0.0841(2) | 1.0 | 0.50(3) | – |
References
(1) I. D. Brown and D Altermatt: Acta Cryst., B41 (1985) 244-247.

