Application Note B-XRD1026
Introduction
Ionic liquids, consisting of molecular cations and anions, are regarded as the third most important liquid following water and organic solvents. Due to their high electrical conductivity, they have especially gained attention in relation to electrochemical applications such as fuel cells or solar cells. However, research continues, as there are still many open questions regarding the relation between their structures and their physical properties. In this application note, a system featuring simultaneous XRD and DSC measurements was used to evaluate the crystal structure of ionic liquids at temperatures around -70°C.
Measurements and results
Mixed solutions of the ionic liquid DEME (N,N-diethyl-N-methyl-N-2-methoxyethylammonium tetrafluoroborate) were prepared by adding either 0.6 mol% or 6 mol% water. Using an attachment that supports simultaneous XRD and DSC measurements, the solutions were first cooled to below -70°C. Their crystallization behavior during heating to room temperature was then observed using both XRD and DSC. The results are as follows: The solution with 0.6 mol% water content crystalized at -70°C, as Figure 1 shows. In contrast, Figure 2 shows that no crystallization of the solution with 6 mol% water content was observed at -70°C, but a cold crystallization occurred during heating at around -50°C. Afterwards, the solution melted at around 0°C. In this way, simultaneous measurements make it easy to observe crystallization and other phase changes, even in the case of materials with complex phase changes caused by slight differences in water content(1)-(3).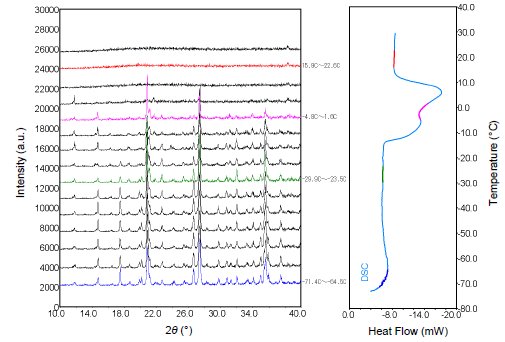 Figure 1: Results of simultaneous XRD and DSC measurements (-70°C→ room temperature) of the mixed solution with 0.6 mol% water content
Figure 1: Results of simultaneous XRD and DSC measurements (-70°C→ room temperature) of the mixed solution with 0.6 mol% water content
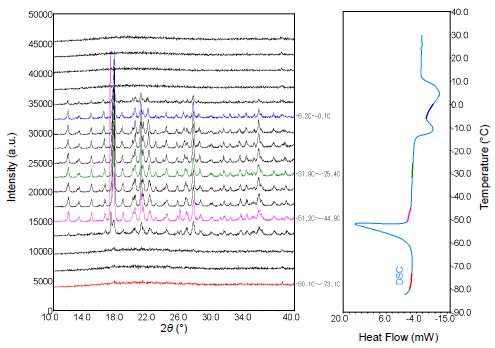 Figure 2: Results of simultaneous XRD and DSC measurements (-70°C→ room temperature) of the mixed solution with 6 mol% water content
Figure 2: Results of simultaneous XRD and DSC measurements (-70°C→ room temperature) of the mixed solution with 6 mol% water content
References
(1) Yusuke Imai, Hiroshi Abe, Takefumi Goto, Yukihiro Yoshimura, Yosuke Michishita and Hitoshi Matsumoto: Chemical Physics, 352(2008) 224-230.
(2) Yusuke Imai, Hiroshi Abe, Takefumi Goto, Yukihiro Yoshimura, Shogo Kushiyama and Hitoshi Matsumoto: The Journal of Physical Chemistry B, 112(2008) 9841-9846.
(3) Yusuke Imai, Hiroshi Abe and Yukihiro Yoshimura: The Journal of Physical Chemistry B, 113(2009) 2013-2018.
Sample provided by: Dr. Hiroshi Abe, National Defense Academy of Japan.

