Battery Material Characterization
Crystal structure analysis is useful for clarifying the identity of the synthesized battery material. For crystal structure analysis, analysis of the average structure by Rietveld refinement and analysis of local structure by PDF analysis is generally used.
BVS ANALYSIS OF LFP AND PDF ANALYSIS OF LMO WITH XRD
1. BVS analysis of LFP with XRD
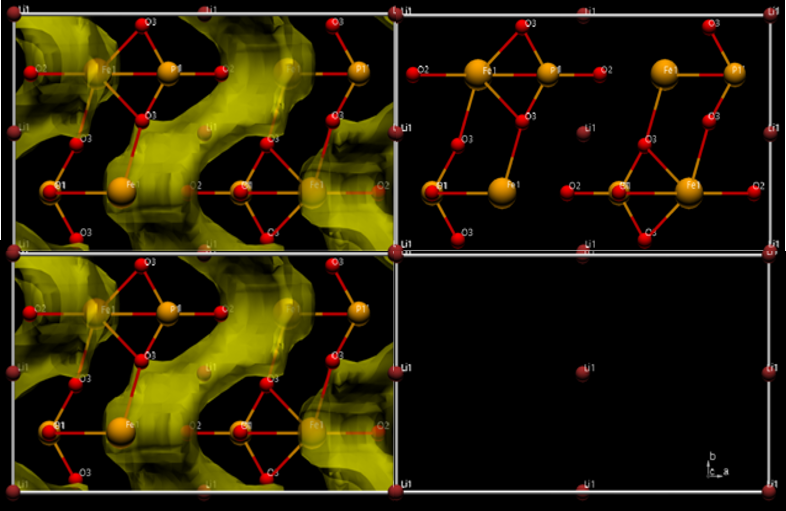
The valence of a positive ion can be evaluated by the BVS (Bond Valence Sum) method utilizing interatomic distances returned by Rietveld refinement. From the valance evaluation of Lithium Iron Phosphate (LFP), the valance of Li was calculated as 1+ and Fe was calculated as 2+. In addition, the diffusion path of the Li-ion (displayed in yellow) can be shown by simulating places where Li-ions tend to become monovalent in the crystal structure of LFP. The BVS method is an effective tool for material design and research into Li battery materials, allowing estimation of the diffusion paths for Li-ions.
LFP (LiFePO4)
Structure analytical result of Lithium Iron Phosphate
|
Element |
x |
y |
z |
Valence |
Bond Valence Sum |
|
Li1(Li) |
0.000000 |
0.000000 |
0.000000 |
1 |
0.977313897861123 |
|
Fe1(Fe) |
0.28231(9) |
0.250000 |
0.9744(3) |
2 |
1.90827098596528 |
|
P1(P) |
0.09511(17) |
0.250000 |
0.4188(4) |
0 |
0 |
|
O1(O) |
0.0948(4) |
0.250000 |
0.7422(8) |
0 |
0 |
|
O2(O) |
0.4563(4) |
0.250000 |
0.20847(7) |
0 |
0 |
|
O3(O) |
0.1638(3) |
0.0495(5) |
0.2848(5) |
0 |
0 |
BVS of Li is 0.98
≒ Univalent
BVS of Fe was 1.91
≓ Divalent
2. PDF analysis of LMO with XRD
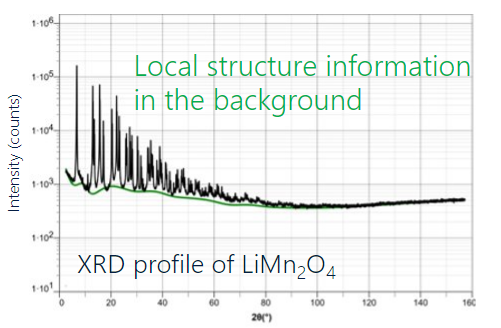
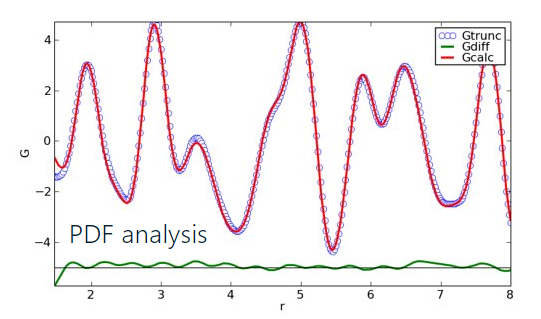
Rietveld refinement is routinely used for crystal structure analysis from powder diffraction data. As with a single crystal structure solution, Rietveld refinement determines an overall average structure, with local deviations from the average structure appearing as average disorder contributions. Pair Distribution Function (PDF) analysis, on the other hand, allows a more detailed investigation of local versus average order and can reveal information concerning changes in packing arrangement as a function of distance between atoms. PDF analysis is a total diffraction technique making use of analytical background intensity, containing local structure information, as well as intensity from diffraction peaks and amorphous halos. As an illustrative comparison between Rietveld and PDF analysis, consider the results for a Lithium Manganese Oxide (LMO) cathode material presented below. When analyzing the X-ray powder diffraction data collected on LMO with cubic as the average structure or with orthorhombic as the average structure, there is no difference in R value when performing Rietveld refinement. Rietveld refinement with a single average crystal structure is unable to differentiate between orthorhombic or cubic average symmetry. Utilizing PDF analysis on the same powder data looking at the relatively short-range atom-atom interactions out to 8Å, there is a significant difference in R-value between cubic and orthorhombic structures, with orthorhombic symmetry giving the better solution. PDF analysis can provide additional insights into the local atomic packing arrangements that are not accessible when utilizing Rietveld analysis.
XRD profile of LiMn2O4
PDF profile of LiMn2O4
Analysis result of LiMn2O4 by Rietveld and PDF refinements
| Crystal System | Rietveld RWP(%) | PDF RW(%) |
| Cubic | 7.0 | 12.4 |
| Orthorhombic | 6.8 | 6.9 |
Related products
SmartLab
Advanced state-of-the-art high-resolution XRD system powered by Guidance expert system software
Learn More
Contact Us
Whether you're interested in getting a quote, want a demo, need technical support, or simply have a question, we're here to help.


/SmartLab%20Studio%20II%20splash%20screen.png?width=800&height=610&name=SmartLab%20Studio%20II%20splash%20screen.png)