Application Note B-XRD1120
Introduction
Organic compounds represented by drug substances sometimes have crystal structures with a low symmetry in molecular arrangement, and dozens of peaks are observed in the X-ray diffraction profile. With a mixture of compounds exhibiting such a complex profile, peak separation is difficult. For this reason, it was also difficult to obtain an accurate value in quantitative analysis that calculates the weight fraction from the integrated intensity of the separated peak. The new quantitative analysis method developed by Rigaku; Direct Derivation method (DD method) calculates the quantitative value based on the single-phase diffraction profile (1) (2). This improves the peak separation accuracy of mixtures that shows complex profiles and makes quantification straightforward. In this application, quantitative analysis of trace amounts of pharmaceutical polymorphic forms was conducted as an example.
Measurements and results
Carbamazepine (CBZ) has crystal polymorphisms of form I (space group: P21/n) and form III (space group: P1). Figure 1 shows the multiple writing of form I and III diffraction profiles. It can be seen that there is a lot of peak overlaps between form I and form III. 1.00 mass% of form III was mixed with form I, and X-ray diffraction for 2θ = 3 to 120° was measured at 10° / min (1 measurement approx. 12 minutes). Figure 2 shows the result of the refinement of the calculated profiles for the obtained profile. Although the Rwp value is high due to the effect of the preferred orientation of the crystals, the quantitative value of 0.99 ± 0.09 mass% for form III was obtained for the preparation value of 1.00 mass%.
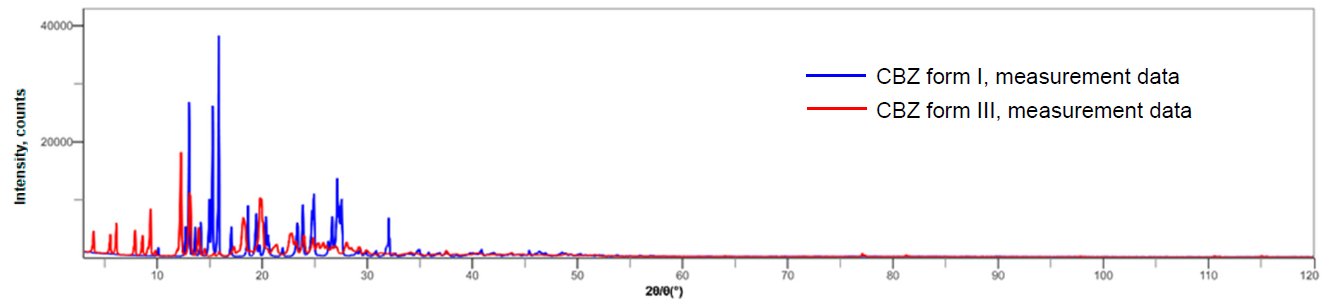
Figure 1: Multiple X-ray diffraction profiles of CBZ form I and form III
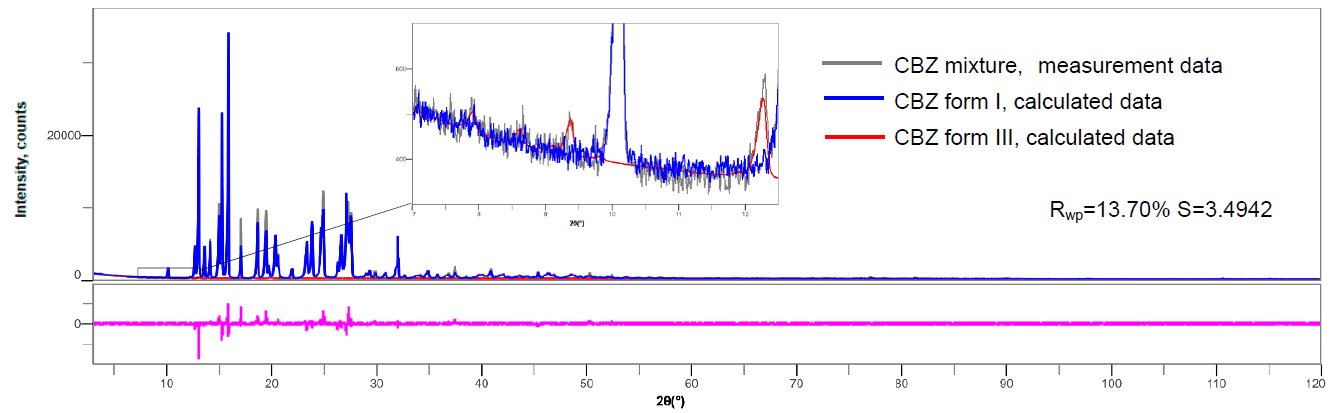
Figure 2: Refinement result of CBZ mixture (99.00% form I and 1.00% of form III were mixed)
References
(1) H. Toraya: J. Appl. Cryst., 49 (2016) 1508-1516.
(2) H. Toraya: J. Appl. Cryst., 50 (2017) 820–829.

