Application Note B-XRD1035
Introduction
The use of single crystals for crystal structure analysis is a common practice. However, it is difficult to apply crystal structure analysis to samples where it is hard to isolate the crystalline pure substance, or for samples that are difficult to crystallize even if a pure substance is obtained. It is becoming possible to perform crystal structure analysis on powder samples if a pure crystalline substance can be obtained. This progress is due to recent increases in the calculation speed of personal computers and significant developments in analysis software, as well as improvements in the precision of powder X-ray diffractometers. In this example, identification of impurities was performed using the diffraction profile of a powder cocrystal sample with impurities. The remaining diffraction peaks were used for indexing, structure determination, and structure refinement.
Measurements and analysis
Although flufenamic acid and salicylic acid cocrystals are obtained from a liquid solution containing flufenamic acid and salicylic acid, it is difficult to obtain single crystals of a size suitable for single crystal X-ray structural analysis. Also, powder (impurity) with only re-crystallized salicylic acid is contained along with the cocrystals. Figure 1 shows the result of determining the crystal structure of the flufenamic acid and salicylic acid cocrystals from the diffraction pattern of the powder sample containing salicylic acid crystals, and refining the crystal structure using the Rietveld method. The results of the analysis showed that the cocrystals contain 1:1 flufenamic acid and salicylic acid, and that two molecules are hydrogen bonded through the carboxyl groups (Figure 2). Moreover, the results showed that the synthesized powder contained about 5.7 mass% of salicylic acid crystals that did not cocrystallize. 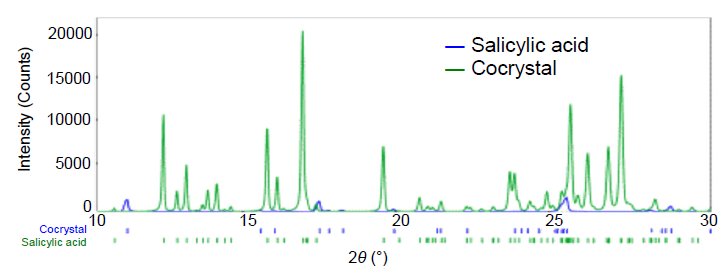 Figure 1: X-ray diffraction patterns of salicylic acid and salicylic acid and flufenamic acid cocrystals
Figure 1: X-ray diffraction patterns of salicylic acid and salicylic acid and flufenamic acid cocrystals
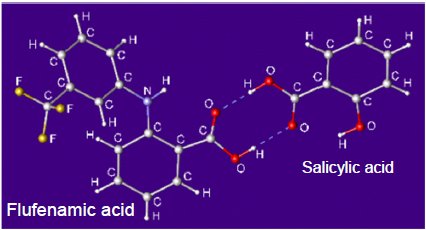 Figure 2: The crystal structure of cocrystals obtained from analysis of the powder sample
Figure 2: The crystal structure of cocrystals obtained from analysis of the powder sample
References
Hisashi Konaka: The 130th Annual Meeting of the Japanese Pharmaceutical Society, (2010), 29P-pm084. Sample provided by: Chugai Pharmaceutical Co., Ltd.

