Application Note B-XRD1141
Introduction
Cement reacts with water to form hydrates, which gradually condense and harden. This hardening is caused when the formed hydrates coat cement particles and bind them together. A detailed understanding of the hydrate formation process is expected to lead to the elucidation of the mechanism of condensation and hardening. Since the X-ray diffraction method is a powerful analytical technique for crystalline phase identification, it can identify crystalline hydrates such as ettringite and monosulfate in cements. In this analysis example, we observed changes in the crystalline phase of ordinary Portland cement over time with a water cement ratio (W/C) of 25%.
Example of measurement and analysis
The measurement was performed using a liquid sample holder. This holder can prevent the sample from drying in an ambient environment by covering the sample surface with a film. Figure 1 shows the changes in the X-ray diffraction pattern of cement during the hydration reaction. Figure 2 shows the change in diffraction peak intensities over time of ettringite (3CaO, Al₂O₃, 3CaSO₄, 32H₂O), monocarbonate (3CaO, Al₂O₃, CaCO₃, 11H₂O) and gypsum (CaSO₄・2H₂O). Ettringite and monocarbonate are hydrates and gypsum is a component of plaster. The gypsum intensity gradually decreased until almost no peaks could be observed. The intensity of ettringite increased for about nine hours and then decreased. Ettringite is a hydrate formed in the early stage of the hydration reaction between the aluminate phase (3CaO, Al₂O₃) and the plaster component. The intensity of monocarbonate began to increase after about two hours and then continued to increase. Monocarbonate is formed by the reaction of the aluminate phase with ettringite and limestone components. This shows that the change in the intensity of ettringite is related to the formation of monocarbonate. The early stage of the hydration reaction affects the condensation of concrete and its initial strength development. In addition, if the formation of ettringite is delayed, the concrete may crack due to expansion. Thus, observing the changes over time in the diffraction peaks of hydrates contributing to the hydration reaction should help gain knowledge about the strength development and expansion of concrete.
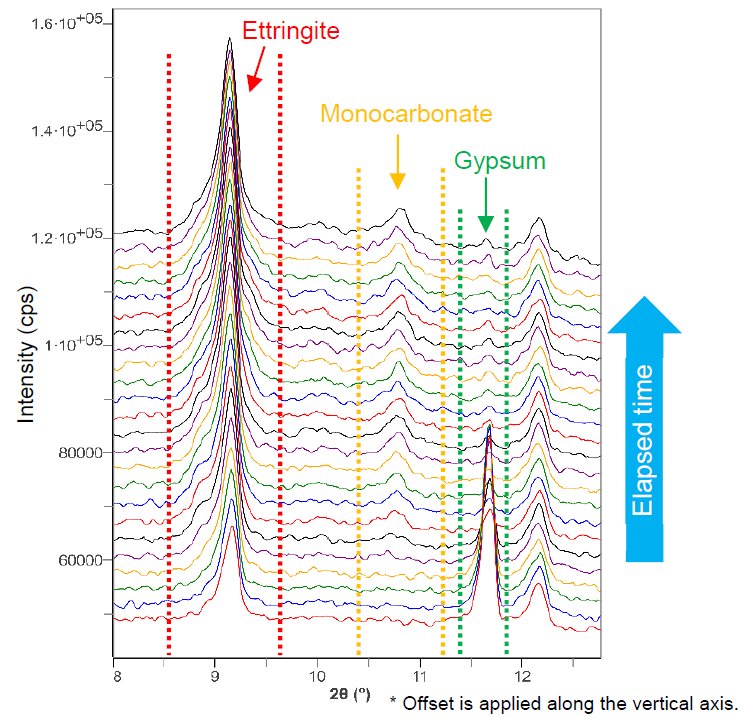
Figure 1: Overlaid X-ray diffraction patterns of hydrated cement
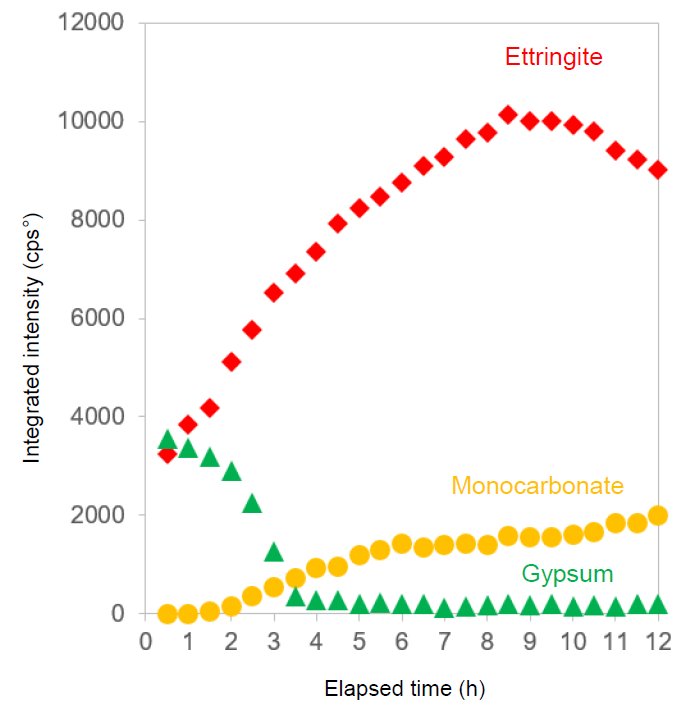
Figure 2: Changes over time in diffraction peak intensity of hydrates in hydrated cement

