Application Note B-TA2019
Introduction
Hydroxyapatite, a calcium phosphate mineral mainly found in bones and teeth, is attracting attention as a material that demonstrates high biocompatibility. Also, product development on optimizing its functionality such as ion exchange and absorbability is actively carried out. Here, we use theTG-MS method to analyze the thermal behavior of various hydroxyapatites produced in various methods.
Measurement and results
Three hydroxyapatite powders namely samples 1, 2 and 3 were used in this experiment. During measurement, 10mg of hydroxyapatite powder weighed in Pt pan was heated from RT~1400℃ at 20℃/min under He flow. The electron ionization (EI) method was performed for MS measurement.
Figure 1 shows the TG and gas evolution behavior (temperature profile of MS signal) in each sample. It could be that during the synthesis of hydroxyapatite, the OH group is replaced with anions or a mixture of impurities resulting to a large difference in evolution behavior of gases. Although Sample 1 exhibited the smallest mass loss compared to other samples, the continuous mass loss can be observed up to 1400℃. In addition to the evolution of H₂O (m/z 18) and CO₂ (m/z 44), NO (m/z 30) and SO₂ (m/z 64) gases were also detected. Mass loss in Sample 2 can be observed at 400℃ and 600 ℃ where H₂O and CO₂ were evolved, respectively. Sample 3 revealed a large mass loss due to the evolution of CO₂ gas near 800℃ in which we were able to confirm the evolution of H₂O, NO and SO₂.
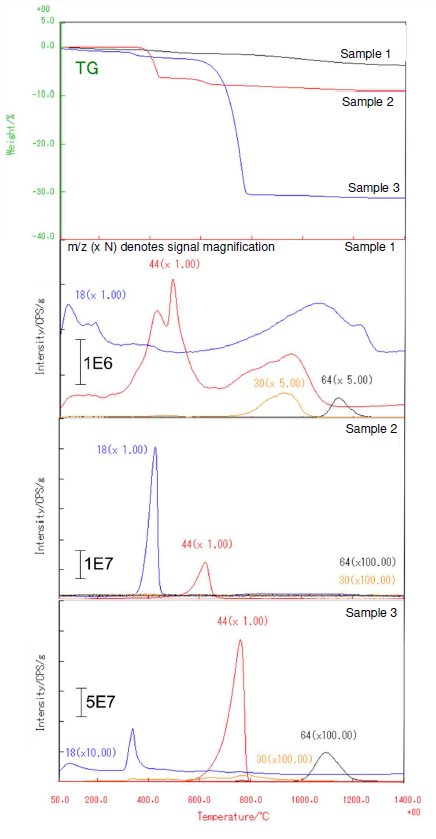
Figure 1: TG and evolution behavior of gases detected from all hydroxyapatite samples.
(The samples were kindly provided by Professor Masato Yamamoto, Showa University, Faculty of Arts and Sciences at Fujiyoshida)

