Application Note B-TA1035
Introduction
Simultaneous thermal analysis (STA) is a method composed of thermogravimetry and differential thermal analysis that simultaneously measures the mass changes associated with endothermic or exothermic reaction. Generally, an open pan is used in STA measurements for volatiles to easily escape during mass losses which usually gives a broad and poorly resolved endothermic peak especially when a continuous dehydration reaction occurs. In this application, we demonstrate the influence of pan shape on the dehydration reaction of α, α-trehalose dihydrate, a widely known material in food and pharmaceutical industry.
Measurements and results
4 mg of standard reagent of α, α-trehalose dihydrate were prepared separately for open pan measurement and pinhole pan measurement. For pinhole pan measurement, a seal pan was used with a small hole on the cover which was bored using a needle prior to sealing. Both samples were individually measured in STA8122 flowing with 200 ml/min air atmosphere and heated up to 230℃ at 10℃/min.
The results are shown in Figure 1. The open pan result reveals a mass loss of 9.02% associated with a broad endothermic peak at 101℃ due to dehydration. On the contrary, the pinhole pan result shows a two-stage dehydration reaction of 2.93% and 5.92% that correspond to two endothermic peaks at 99℃ and 121℃ which are both due to dehydration. The latter result shows a sharper and better resolved dehydration phenomena which are shifted slightly to higher temperature. In this case, water is not released from the pan until a sufficient vapor pressure has built up to eliminate the water molecules through the very small hole on the seal cover of the pan. This implies the use of self-generated atmospheric effects to reveal the existence of different water-bonding states within the crystal. However, the endothermic peaks and the peak top temperatures are dependent on the size of the pinhole and measurement conditions such as heating rate, sample amount as well as particle size. Therefore, it not recommended to use this method when analyzing the onset temperature of the dehydration reaction and precautionary measures needs to be fully considered. Furthermore, both results also indicate that the endothermic peak at 208℃ due to melting is not affected by sample pan shape.
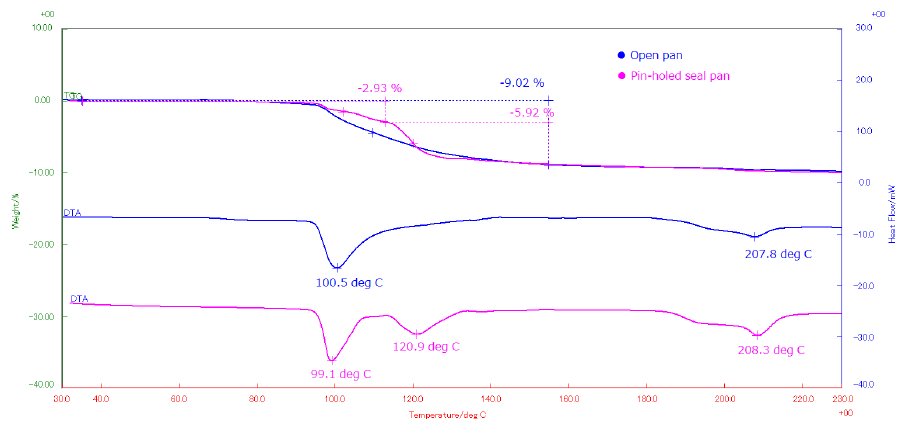
Figure 1: Comparison of STA measurement data of α, α-trehalose dehydrate between open pan (blue) and pinhole pan (pink)
Reference
(1) L.S. Taylor and P. York. J. Pharmaceutical Sciences 87-3 (2000) 347-355

