Observation of Dehydration Behavior of a Drug Substance using TG-DTA and XRD-DSC
Introduction
Hydrate crystals maintain their structure when water is taken into the crystal, but the crystal structure changes or becomes amorphous when the crystal water is lost. There is a correlation between crystal structure and physical properties, and it is important to understand the phase transition behavior due to changes in humidity when controlling the quality of hydrates. Measuring the weight reduction of the sample while heating the hydrate by TG-DTA, the amount of desorbed water can be determined. In addition, if the hydrate is heated and the X-ray diffraction is measured by XRD-DSC, the dehydration temperature and the state after dehydration can be determined. Here, we investigated the dehydration behavior of a hydrated drug substance using TG-DTA and XRD-DSC.
Measurements and results
The antiallergic drug nedocromil sodium (NS) is known to take amorphous, anhydride, monohydrate and trihydrate. Figure 1 shows the TG-DTA measurement results of NS trihydrate. It was found that the first weight reduction is equivalent to two molecules of water and the second weight reduction is equivalent to one molecule of water, per molecule of drug substance.
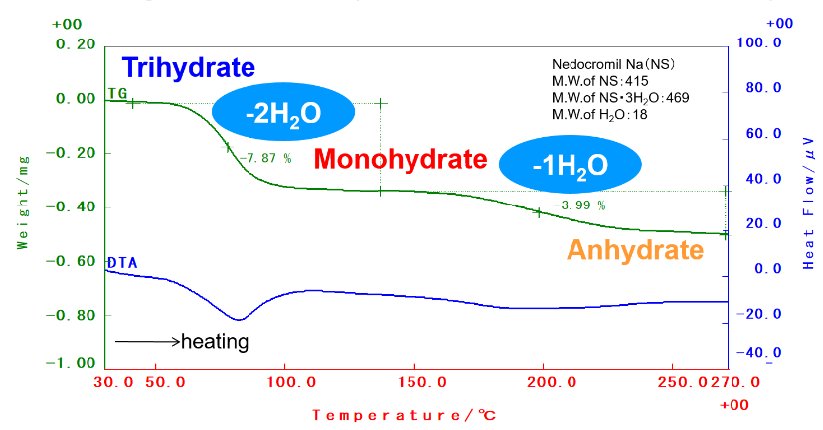
Figure 1: TG-DTA measurement result of NS trihydrate
Figure 2 shows the XRD-DSC measurement results of NS trihydrate measured in N₂ gas (27°C, 3 %RH) atmosphere. The XRD profile changed at the endothermic peak around 70°C, corresponding to the first weight reduction, and 170°C, corresponding to the second weight reduction, respectively. From the results of TG-DTA and XRD-DSC, it was found that the first change in TG-DTA is a crystal phase transition from trihydrate to monohydrate, and the second change is a crystal phase transition from monohydrate to anhydride.
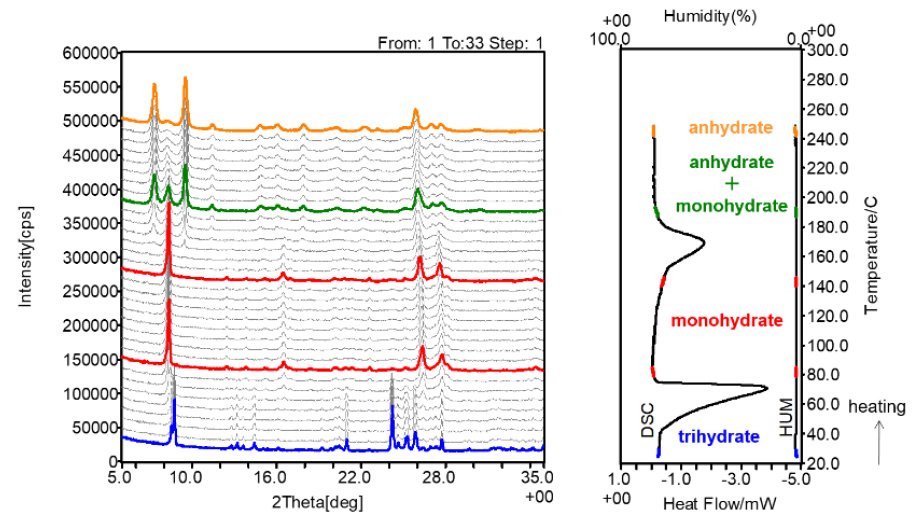
Figure 2: Results of simultaneous XRD-DSC measurement of NS trihydrate.
Samples provided by: Dr. Katsuhide Terada, Toho University

Contact Us
Whether you're interested in getting a quote, want a demo, need technical support, or simply have a question, we're here to help.
