Application Note B-TA2027
Introduction
Polyoxymethylene (POM) also known as polyacetal is an all-purpose engineering plastics with excellent mechanical properties. Due to its durability, it is used as a substitute for metal parts mainly used as gears, bearings, various parts. Other uses include stationery and musical instruments such as woodwind and brass instruments. Evolved gas analysis is used to qualitatively confirm the evolved gases as POM is heated.
Measurement and results
POM was measured in EI mode of Thermo Mass Photo, heating from RT~500℃ at 20℃/min in He atmosphere. Figure 1 shows the multiplot of TG-DTA measurement results and MS ion thermograms. A two-stage mass loss is observed from 320~420℃ on the TG curve. The endothermic peak seen at 173℃ is due to melting, while the endothermic peaks at 378℃ and 403℃ are due to thermal decomposition of POM. The main detected peaks were m/z 29, 30 indicating formaldehyde as the main composition of the evolved gases. This result further reveals that POM is a polymer of formaldehyde that decomposes to generate monomers as evolved gases.
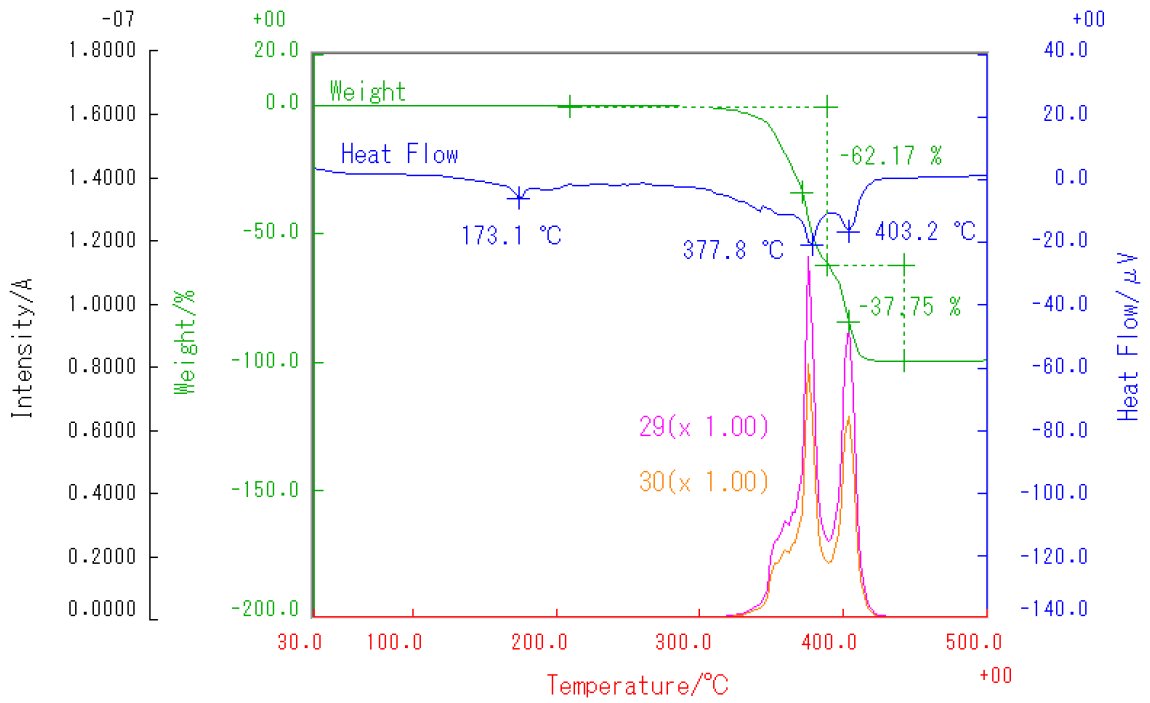
Figure 1: TG-DTA and MS ion thermogram(m/z 29,30)
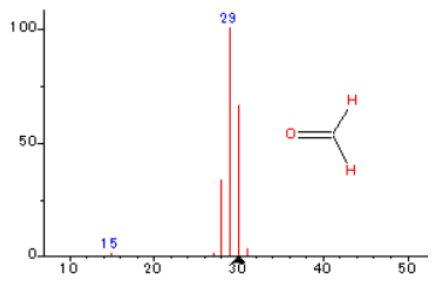
Figure 2: Formaldehyde mass spectrum (NIST Lib)
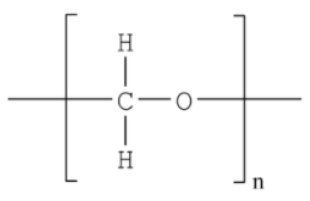
Figure 3: Structural formula of POM

