Application Note B-TA1037
Introduction
Simultaneous thermal analysis (STA) is a hyphenated technique composed of thermogravimetry (TG) and differential thermal analysis (DTA). TG is a method in thermal analysis that evaluates the mass changes as the material is heated or cooled while the DTA monitors the temperature difference between the sample and the reference which are revealed as endothermic or exothermic reaction. Recently, sample observation in thermal analysis measurement has increasingly becoming significant in STA and DSC measurements, providing a more detailed assumption. Using this function, we can elucidate the thermal behavior and confirm the visual changes in shape and color that occur in a sample during a reaction. Here, we evaluate aspartame with sample observation STA. Aspartame is used in chewable tablets and sugar-free liquids in pharmaceutical industry replacing sugar.
Measurement and results
Reagent grade weighing 5 mg aspartame placed in an aluminum crucible was measured in an STA equipped with sample observation option heating from 30℃ up to 500℃ at 20℃/min in N₂ flowing at 300 ml/min. STA results with snapshots of actual sample images in arbitrarily selected temperatures are shown in the figure below. STA results revealed a three-stage mass losses on the TG curve. The mass loss with endothermic reaction at 124℃ is due to dehydration, while the mass loss with endothermic reaction at 192℃ is attributed to the loss of methanol and the formation of diketopiperazine which melted at 250℃. This was followed by the decomposition of diketopiperazine from 300℃. The sample images revealed less change in shape and color up to 224℃ and drastic changes in color and shape were observed during the melting and thermal decomposition reactions.
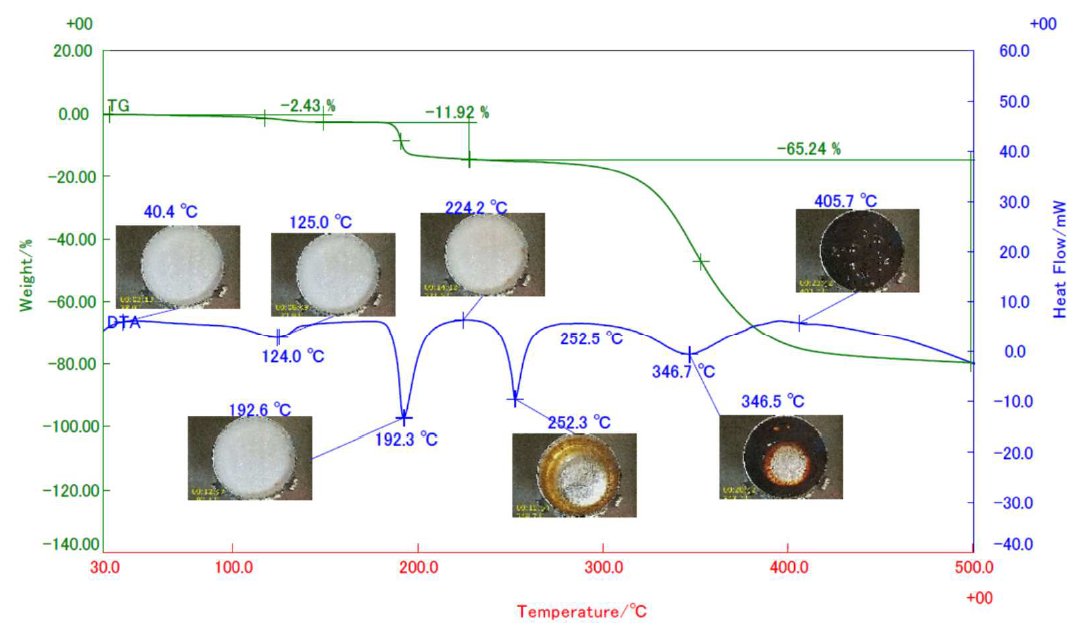
Figure 1: Thermal behavior of aspartame by sample observation STA

