Application Note B-TA1071
Introduction
Wood is composed of polysaccharides such as cellulose, hemicellulose, and lignin, etc, and is used in a wide range of applications, including building materials, furniture, musical instruments, and tableware, as well as being used as a raw material for pulp.
It is generally known that wood decomposes and combusts at temperatures above 200°C. In this study, the combustion behavior was compared under different humidity-controlled conditions.
Measurement and analysis example
A piece of wood used for disposable chopsticks was cut into a block of approximately 5 mg, placed in an aluminum pan, and STA measurements were conducted at 10℃/min under dry air and atmospheric humidity-controlled air at 90% RH.
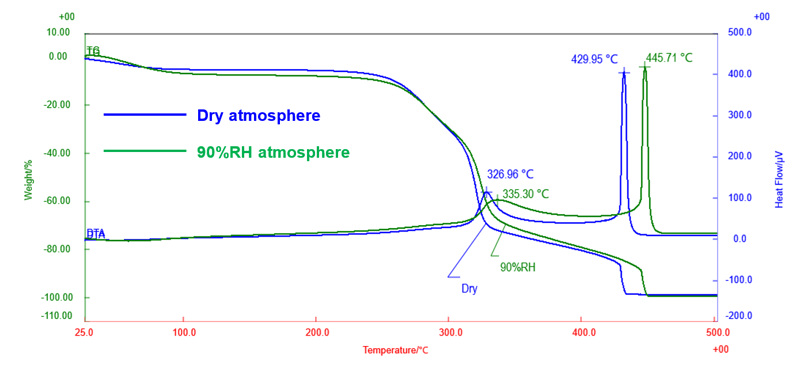
Figure 1: Comparison of the TG-DTA results
In both dry and 90%RH air atmospheres, mass loss attributed to be dehydration was observed up to around 200°C. Subsequently, approximately 25% mass loss was observed up to around 300°C, followed by approximately 48% up to around 400°C. Nearly the entire amount of sample had decomposed by around 450°C.
The mass losses in the ranges of 300°C to 400°C and 400°C to 450°C were accompanied with exothermic peaks, which suggests that the mass loss and exothermic reaction were due to combustion. When comparing the onset temperatures of mass loss and the exothermic peak, these values were shifted toward higher temperatures in the 90%RH atmosphere compared to those of the dry atmosphere, indicating that combustion occurs at higher temperatures under high-humidity conditions.
Based on the above results, it is suggested that it is important to consider the effect of atmospheric humidity when evaluating the heat resistance of woods.

