Application Note TA6022
Introduction
Polyetheretherketone (PEEK) is an aromatic polyketone and commonly used as an engineering thermoplastic materials composed of ketone and aromatic moieties with a high thermal stability, chemical and radiological resistance. The molecular structure of PEEK is shown in Figure 1. It is used in many high performance applications due to its properties, such as in implant materials, aerospace, automotive industries, power and energy generation. In this application, we study the thermal decomposition of PEEK in inert and oxidized atmosphere by Thermo Mass Photo.
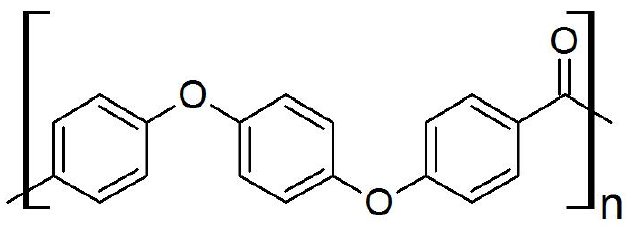
Figure 1: Molecular structure of PEEK
Instrument: Thermo Mass Photo
Thermo Mass Photo is a simultaneous hyphenated technique involving thermogravimetry-differential thermal analysis (TG-DTA) and mass spectrometry (MS) interfaced with a skimmer-type interface. The skimmer-type interface is located inside the furnace near the sample allowing the transfers of gas efficiently. It has the same environmental temperature with the measurement sample, thereby responsible for the real-time measurement as a function of temperature or time. The Thermo Mass Photo is further equipped with a standard electron ionization (EI, 70 eV) method for the confirmation of fragmentation pattern; and a soft ionization method, photoionization (PI, 10.2 eV), for the detection of molecular ions without fragmentation. Therefore, the Thermo Mass Photo is an indispensable tool for basic and advanced research, development of new materials, quality control, etc.
Experimental
1~2 mg of natural grade PEEK (450G) manufactured by Victrex were placed in platinum pans. Prepared samples were heated at 20℃/min under 100% He for inert atmosphere; and He/O₂ (80%/20%) for oxidized atmosphere from room temperature up to 950℃. Their thermal behaviors as well as evolved gaseous prod-ucts under inert and oxidized atmospheres were respectively analyzed by TG, DTA and MS.
Results and Discussion
Figure 2 shows the TG and DTA of PEEK measured under inert and oxidized atmospheres. The results measured under inert atmosphere indicate mass losses initiating at 583℃ with a total mass loss is 53% and that the remaining polymer mass is carbonaceous char that cannot be thermally decomposed under inert atmosphere. On the other hand, measurements under oxidized atmosphere reveal mass losses observed from 514℃ associated with exothermic reactions indicating combustion and that the total mass loss is 100%.
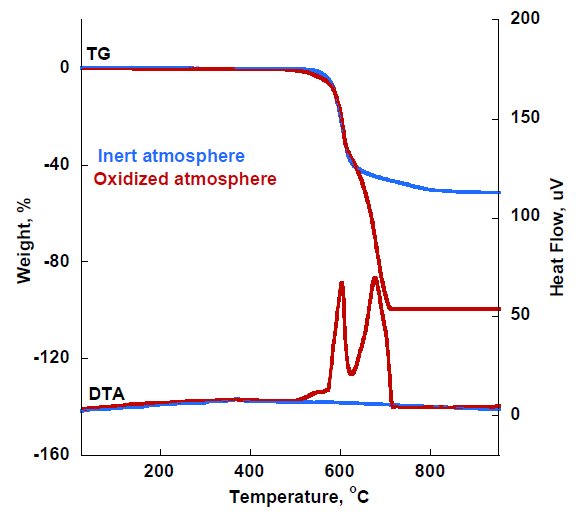
Figure 2: TG-DTA of PEEK measured under inert and oxidized atmosphere
Figure 3A and Figure 3B shows the mass spectrum at 600℃ by EI-MS under inert atmosphere. Results indicate that the main thermal decomposition products are m/z 18, 28, 44 and 94. Other detected ions from EI-MS are m/z 78, 108, 115, 139, 141, 168 and 170. Due to simultaneous evolution of decomposition products as well as due to excessive fragmentation in the EI-MS, we can only deduce that the main decomposition products derived from PEEK under inert atmosphere are water (H₂O, m/z 18), carbon monoxide (CO, m/z 28), carbon dioxide (CO₂, m/z 44) and phenol (C₆H₆O, m/z 94); and that other detected ions are speculated as benzene (C₆H₆, m/z 78), dibenzofuran (C₁₂H₈O, m/z 168) and diphenyl ether (C₁₂H₁₀O, m/z 170). Other dominant peaks in the mass spectrum are m/z 141, 115 and 139, 113 which are considered as fragment ions from diphenyl ether (mw 170) and dibenzofuran (mw 168), respectively. 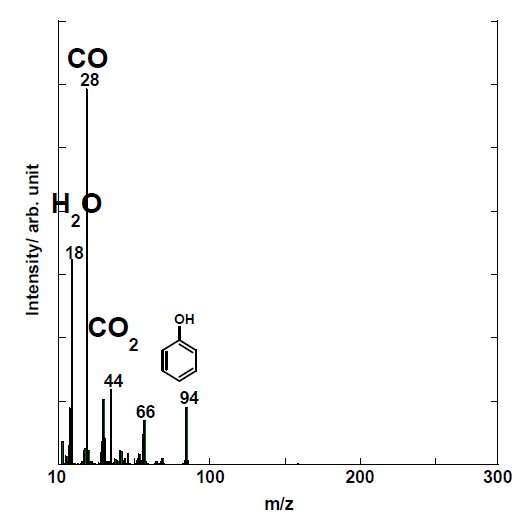
Figure 3A: EI-MS spectrum of PEEK at 600℃ under inert atmosphere
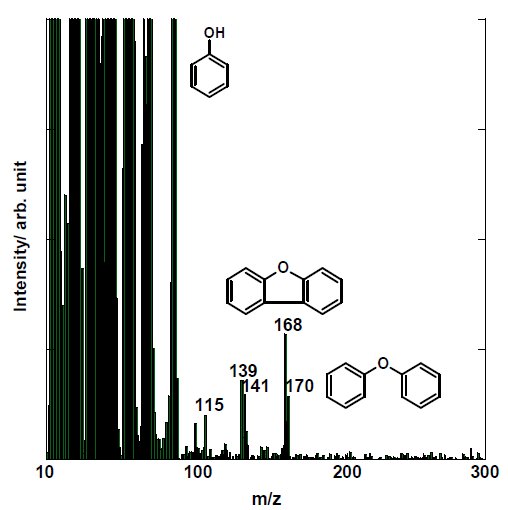
Figure 3B: (Zoom). EI-MS spectrum of PEEK at 600℃ under inert atmosphere
Under oxidized atmosphere, the evolved gases observed are mainly m/z 18, 44 and minute amounts of m/z 7 8 by EI-MS which are water, carbon dioxide and benzene. Figure 4A and Fig4B respectively compare the MS ion thermogram of the evolved gases from PEEK by EI-MS measured under inert (straight line) and oxidized (broken lines) atmosphere.
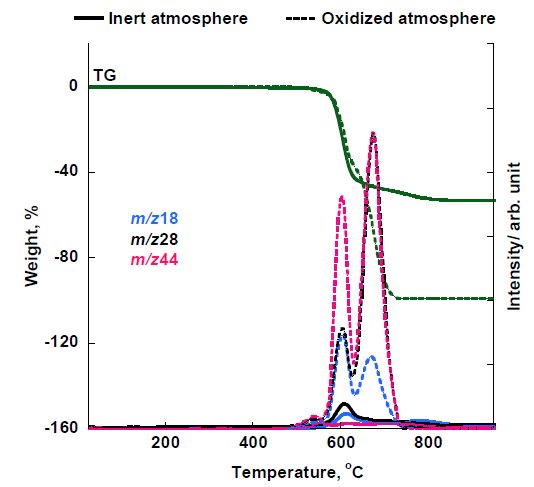
Figure 4A: MS ion thermogram of the thermal decomposition products of PEEK measured by EI-MS under inert and oxidized atmosphere
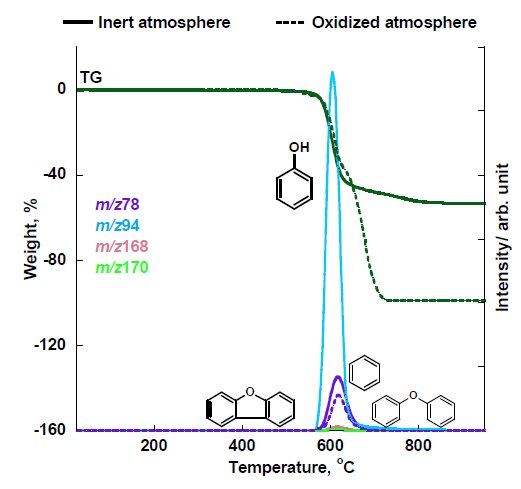
Figure 4B: MS ion thermogram of the thermal decomposition products of PEEK measured by EI-MS under inert and oxidized atmosphere
Results suggest that concentrations of H₂O and CO₂ during combustion are higher compared to inert atmosphere. Also low concentration of benzene (m/z 78) is evolved in oxidized atmosphere and that phenol and other aromatic ethers cannot be observed. These results further reveal that during combustion, evolution of carbon containing compounds were observed as well as the loss of carbonaceous char leading to the increased amounts of CO₂, H₂O; and the loss of phenols and aromatic ethers.
The MS ion thermogram of PEEK measured under inert atmosphere by PI-MS are shown in Figure 5A and Figure 5B (zoom). The PI measurement results indicated that the parent ions detected are m/z 78, 94, 108, 168, 170, 182, 242 and 262 in which some of these parent ions were confirmed in EI-MS results such as benzene (m/z 78), phenol (m/z 94), dibenzofuran (m/z 168) and diphenyl ether (m/z 170). Other detected parent ions can be estimated as benzophenone (m/z 182), 4-4’-dimethoxybenzophenone (m/z 242) and 1,4-diphenoxybenzene (m/z 262).
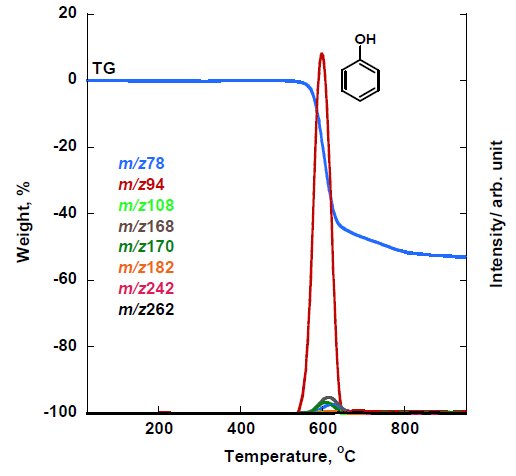
Figure 5A: MS ion thermogram of PEEK measured by PI-MS under inert atmosphere
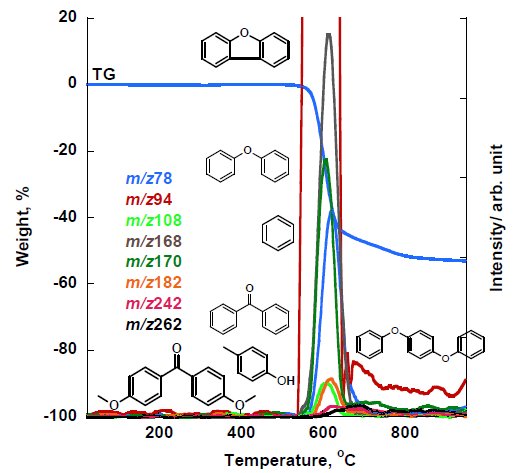
Figure 5B: (Zoom). MS ion thermogram of PEEK measured by PI-MS under inert atmosphere
On the other hand, the MS ion thermogram of PEEK measured under oxidized atmosphere by PI-MS is shown in Figure 6 which reveals that only m/z 78, estimated as benzene, was detected and that other parent ions found under inert atmosphere were not detected under oxidized atmosphere indicating the loss of phenols and other ethers of PEEK during combustion.
Based on the molecular structure of PEEK in Figure 1, the PI measurement results lead to the conclusion that most of these evolved gaseous products are due to random chain scission of the ether and ketone bonds by thermal cleavage; and some products could be due to recombination of adjacent radicals when measured under inert atmosphere.
Furthermore, the Thermo Mass Photo (TG-DTA/ EI- and PI-MS) measurement results clearly demonstrated the real-time change in thermal behavior as well as their respective evolved gaseous products when a polymer is measured under inert and oxidized atmosphere.
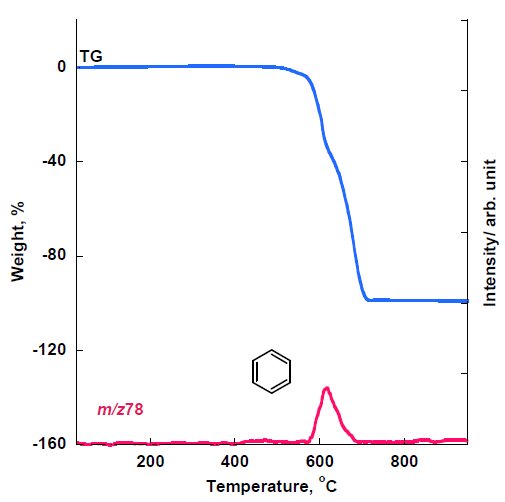
Figure 6: MS ion thermogram of PEEK measured by PI-MS under oxidized atmosphere

