Application Note B-TA1014
Introduction
A binary alloy, such as Sn-Pb solder, is composed of two metals that has metallic properties. These binary alloys can be characterized as heterogeneous alloy or a homogeneous alloy depending on their metal concentration. A eutectic alloy is a homogeneous mixture of substances that melts at a single temperature known as eutectic point, which is lower than the melting points of these individual components. A heterogeneous alloy is composed of two or more phases. Here, we use the DSC method to determine the eutectic temperature and to characterize binary alloys.
Measurements and results
In this measurement, 5 mg high purity Sn, Pb and alloys with different concentrations of Sn and Pb were placed in a crimp pan and was heated at 5℃/min from room temperature up to 340℃ in air static atmosphere.
The DSC measurement results of Sn-Pb 0-100% (①), 50-50% (②), 62-38% (③), 80-20% (④), 100-0% (⑤) are shown in Figure 1A. The respective melting temperatures of all alloys are plotted in the phase diagram shown in figure B. These results reveal that the melting of the binary alloy decreases with decreasing concentration of high purity metal. It also shows that the initial melting temperatures of all alloy samples were observed near 183℃.
In addition, the 62-38% (③) Sn-Pb is a homogeneous alloy and that the eutectic point is observed at 182.9℃. On the other hand, alloys ④ and ② can be identified as heterogeneous alloys composed of liquid and solid phases. Using the phase diagram in Figure 1B, we can conclude that both components are in solid phases below the eutectic point; and that both components are in liquid phases above the melting temperature of the larger concentration. Thru DSC method, we are able to characterize alloys based on their melting properties.
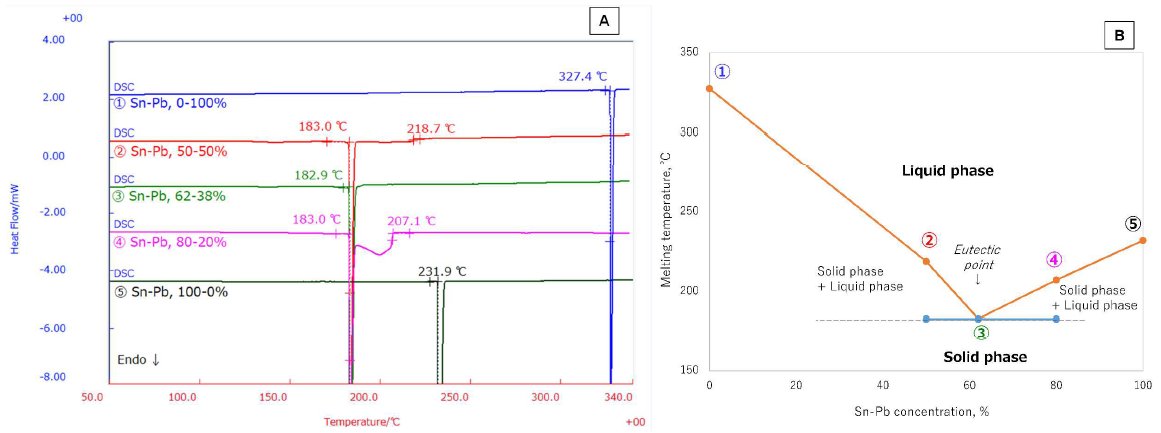
Figure 1: DSC measurement results of high-purity Sn, Pb and alloys with different Sn-Pb concentration (A) and phase diagram of their melting temperatures (B)
Reference
(1): T. Kimura, Training course on Phase Diagrams, 33rd Short Courses in Thermal Analysis (1997), 62-69 (Japanese)

