Pharmalytical Summit 2021
Drug Manufacturing & QC
Drug Product Manufacturing
Material Identification and Verification
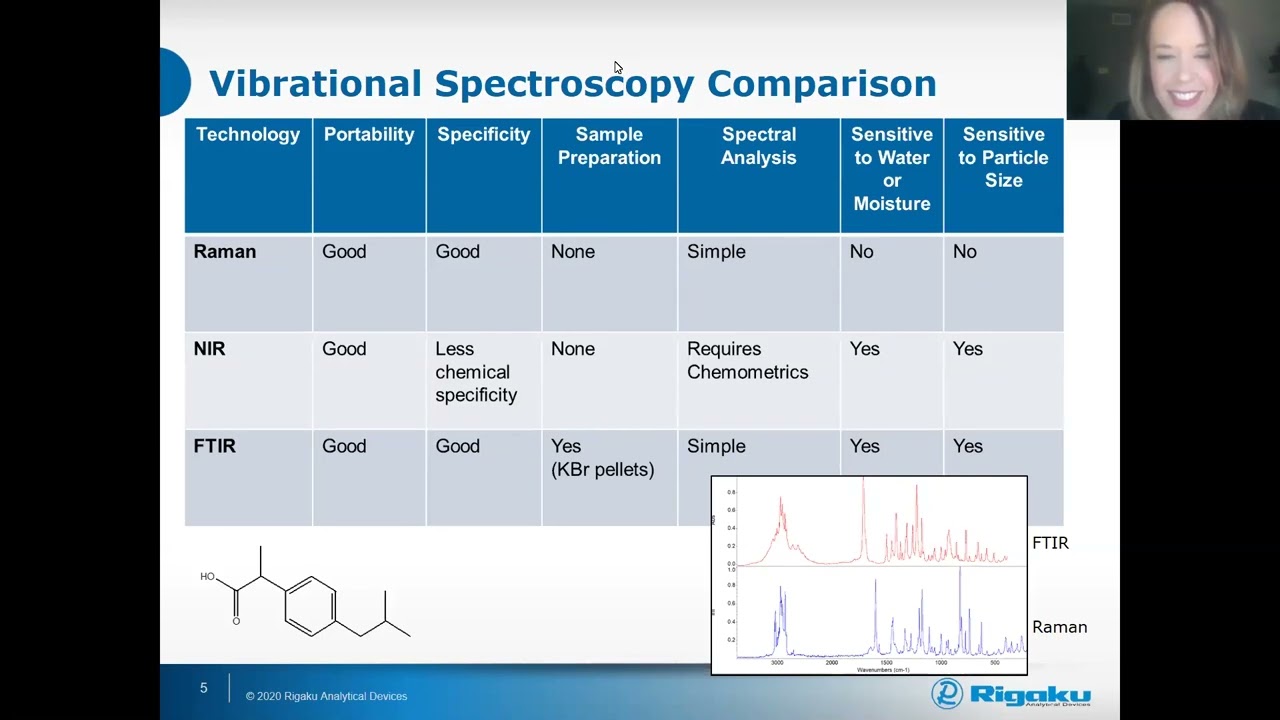
Regulatory drivers governing the modern pharmaceutical manufacturing process are ever-changing. Quality control leaders will be continually pressed to improve quality reach while maintaining leanness. Future-readiness in the globalized pharmaceutical industry has never been more important than it is now. Join us to learn how 1064 nm Handheld Raman offers the proven robustness required of high-throughput raw materials testing while being uniquely versatile enough to provide you the scientific answers you need in more experimental applications. Hear about how Progeny fits into your Data Integrity and Compliance solution puzzle, and learn more about interesting applications like Polymorph speciation and cell culture media analysis.
Non-Destructive 3D Imaging Study Of Tablets and Coatings with X-Ray Computed Tomography
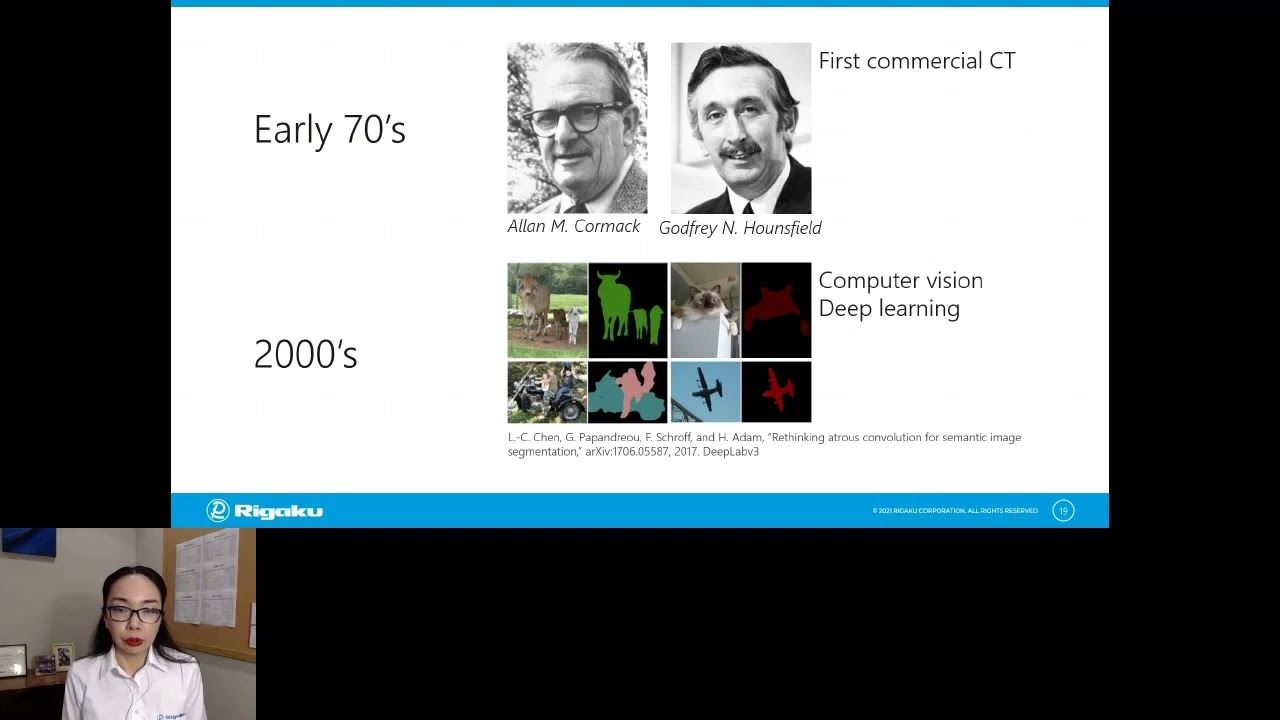
X-ray computed tomography (CT), especially when combined with low energy radiation and high-resolution geometry, can image the 3-dimensional structure of tables non-destructively. This technique can be used to analyze non-uniform mixing of API, aggregates, cracks, and voids in tablets and coating thickness and delamination distributions. X-ray CT analysis can provide insight into why some tablets have a short shelf life, how and why the dissolution process does not have as designed. The level of microstructure detail revealed by X-ray CT can be considered to be a critical aspect of pharmaceutical equivalence for final dosage forms. Basics of X-ray CT and a number of application examples will be presented.
Quality control of API potency, excipient blend uniformity, and heavy metals impurities by non-destructive and direct analysis of intact pills by XRF
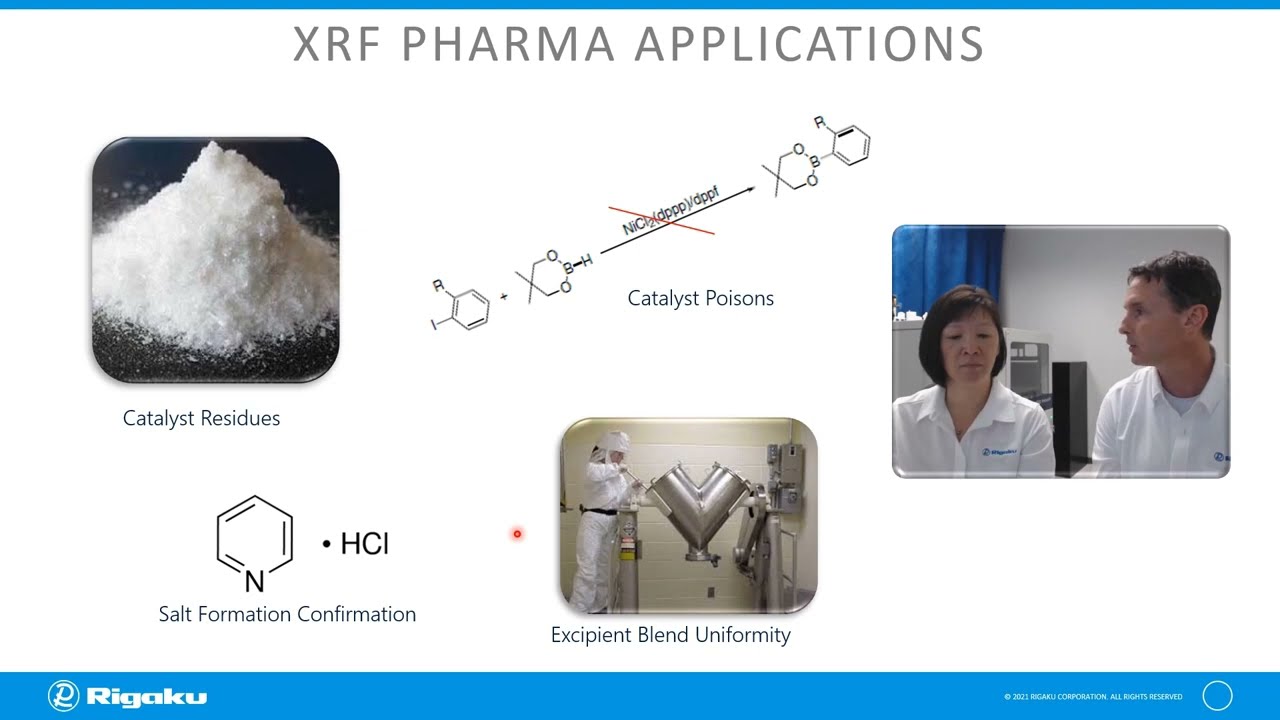
XRF analyses show comparable concentrations to ICP-MS for Class 1 and 2a heavy metals by ICH-Q3D (USP<232>). We will demonstrate quality control of API potency, excipient blend uniformity, heavy metals impurities and the advantages of this non-destructive quality control ready technique for final product testing. In addition, we will discuss the usefulness of XRF in control of manufacturing processes (i.e. blending, tableting, etc.).
The Application of Chemometric and Statistical Analysis Techniques for X-ray Diffraction Data: Quantitative Analysis and Lot Release
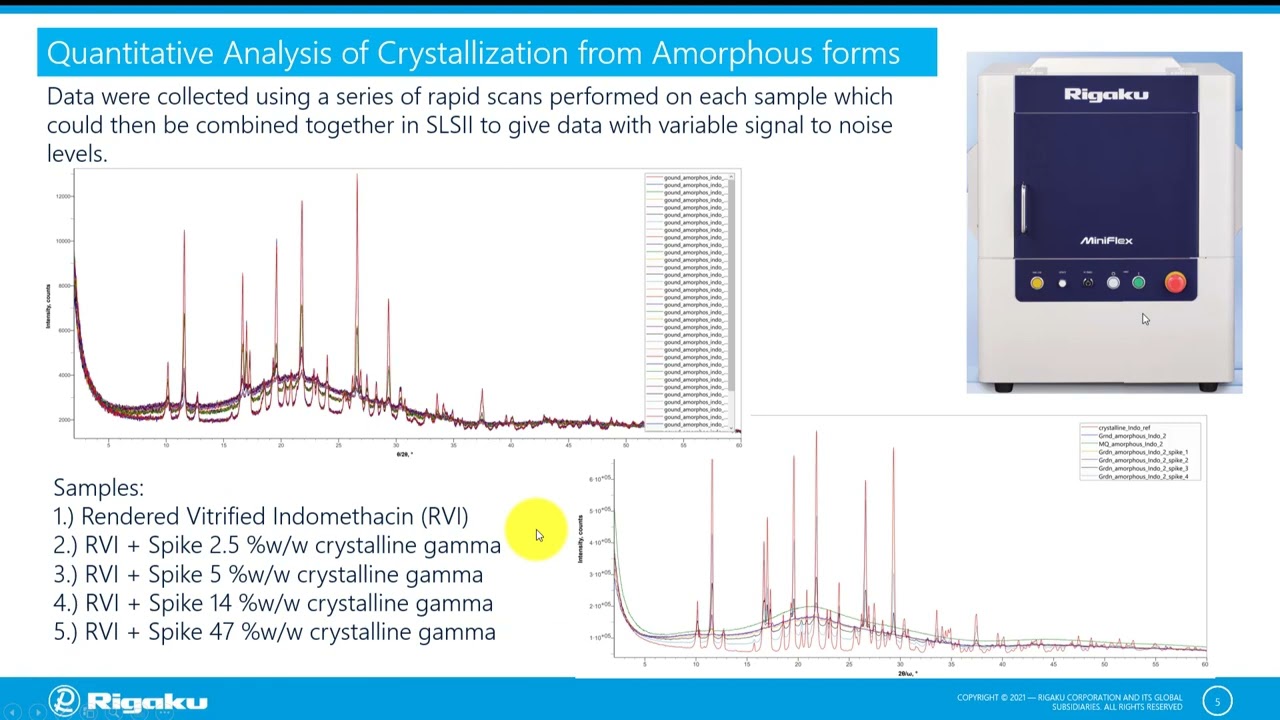
Traditionally, X-ray powder diffraction has used peak based methods for quantitative analysis. Peak based quantitative methods often require extensive calibration and can be inherently flawed for phases that exhibit preferred orientation. More recently, whole pattern fitting using Rietveld type analysis has promised a standardless approach to quantitative analysis and an ability to model preferred orientation. But these advantages often come with increased instability for limit test type methods. In this presentation, a novel Chemometric and total diffraction approach to standardless quantitative analysis and robust lot release methods will be discussed.
Verification of Finished Drug Products Using 1064 nm Handheld Raman

Contact Us
Whether you're interested in getting a quote, want a demo, need technical support, or simply have a question, we're here to help.

Subscribe to the Bridge newsletter
Stay up to date with materials analysis news and upcoming conferences, webinars and podcasts, as well as learning new analytical techniques and applications.