Pharmalytical Summit 2021
Drug Formulation
Brief Discussion on the Science of Equivalences: Rationale and Analytics for Pharmaceutical Equivalence
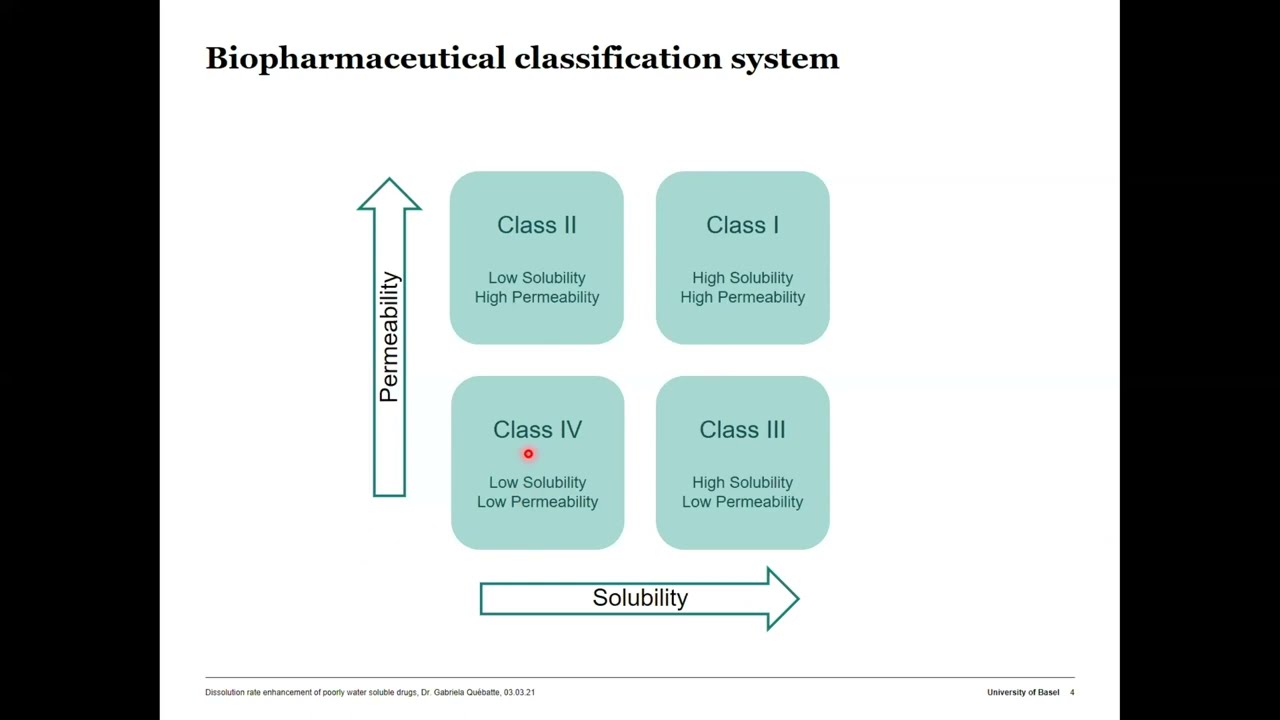
Dissolution Rate Enhancement of Poorly Water Soluble Drugs - Role in XRPD in the Pharmaceutical Formaulation Development
Material Analysis: Combined XRD-DSC for Pharmaceuticals
X-ray powder diffractometer (XRPD) and X-ray single crystal diffractometer are known and used in pharmaceutical research, formulation, and manufacturing. However, it is not well-known a modern laboratory XRD has more advanced options, which provide unique information compared to XRPD. In this presentation, differential scanning calorimetry (DSC) combined with XRPD and Ka1 Johansson configuration for indexing application will be discussed.
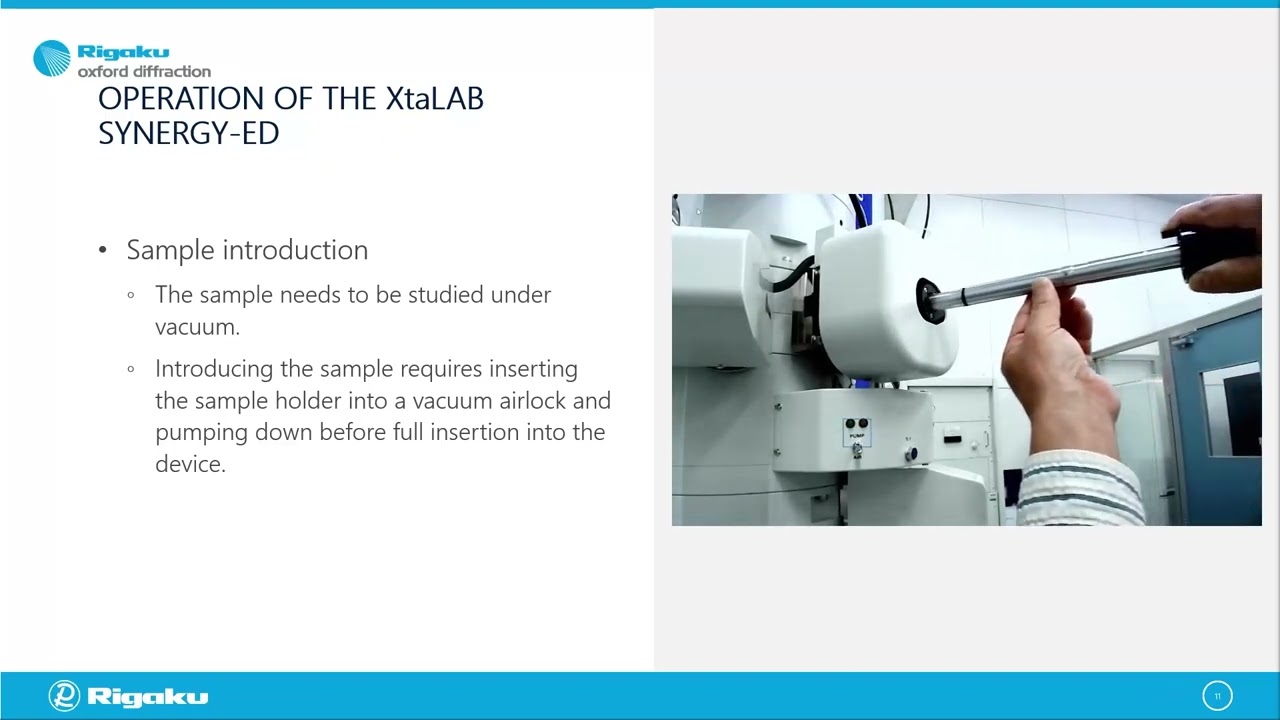
MicroED: An Update from the Rigaku/JEOL Collaboration
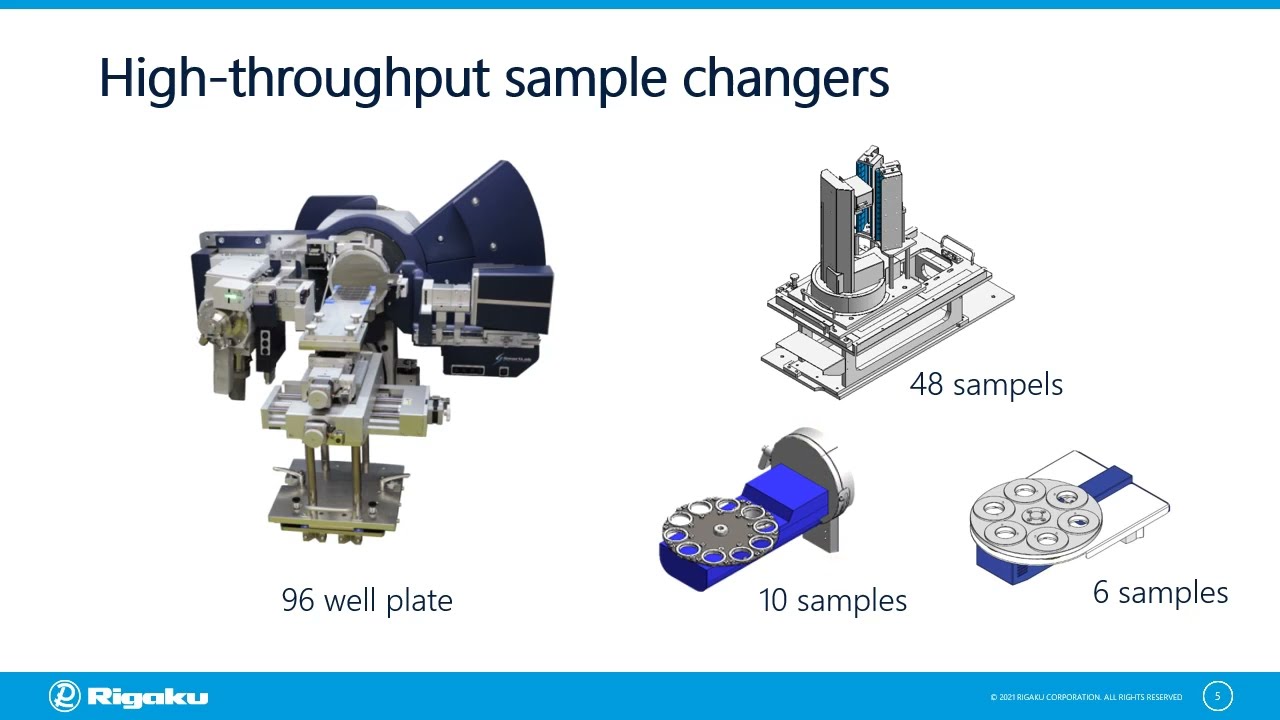
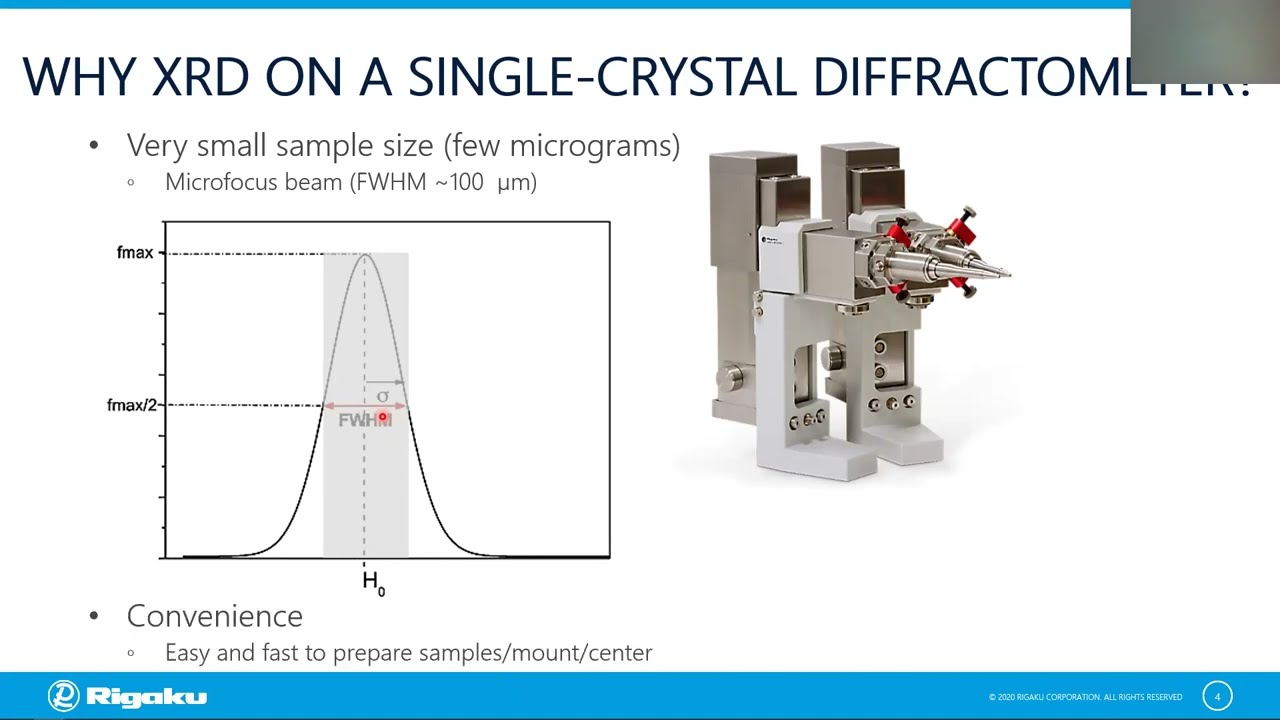
Routine Powder XRD Measurement with the XtaLAB Synergy-S Single Crystal Diffractometer
Despite being an X-ray diffractometer designed for single crystal crystallography, the XtaLAB Synergy-S is also a great tool for the measurement of powder samples. The following features are of particular interest to the pharmaceutical industry:
- Data collection on tiny amounts of sample.
- Use of Gandolfi strategies for data collection, so data is collected with several orientations of the powder holder in the beam.
- Better statistics on weak data, via the direct photon counting technology used by the Rigaku HyPix detectors.
- Improved peak resolution, thanks to the single-pixel point-spread-function of the HyPix detectors and a computer-controller beam divergence slit.
- Batch screening of up to 96 powder samples without user intervention in between samples, using an in-situ plate screener directly mounted on the goniometer.
In this presentation, explanations will be given about how the above-mentioned features are achieved and some data will be presented.
The Use of Easy XRF Elemental Analyses Techniques to Support the Development of Pharmaceutical Formulations
We will discuss fast, quantitative elemental characterization of excipients and API’s via a simple XRF analysis which can be used to check drug product potency, blend uniformity, and heavy metals impurities. We will demonstrate how this technique can be routinely used in an open-access environment.

Contact Us
Whether you're interested in getting a quote, want a demo, need technical support, or simply have a question, we're here to help.

Subscribe to the Bridge newsletter
Stay up to date with materials analysis news and upcoming conferences, webinars and podcasts, as well as learning new analytical techniques and applications.