Calculation of Molecular Stacking Spacing of Copper Phthalocyanine using PDF Analysis
Introduction
Phthalocyanine (Pc) compounds, organic small molecule materials, have excellent electrical properties and durability, and are widely used as organic semiconductor materials and blue paint for bullet trains. A Pc molecule coordinates to a metal ion at the center, displaying various optical and electrical properties. This molecule forms a column-like packing structure in which molecules are stacked on top of each other, showing uniaxial electrical conductivity in the direction of molecular stacking. Electrical properties depend on the molecular stacking spacing, but it cannot be estimated by standard powder X-ray diffraction. On the other hand, PDF analysis can calculate interatomic distance of heavy elements, which may be effective for calculating the distance between metals in the center of Pc molecules (1).
Measurements and analysis
Figure 1 shows a schematic representation of the molecular structure and the molecular packing structure of copper phthalocyanine (CuPc). The powder X-ray diffraction profile was measured using CuKα and the reflection method, and the PDF pattern was measured using AgKα and the transmission method.
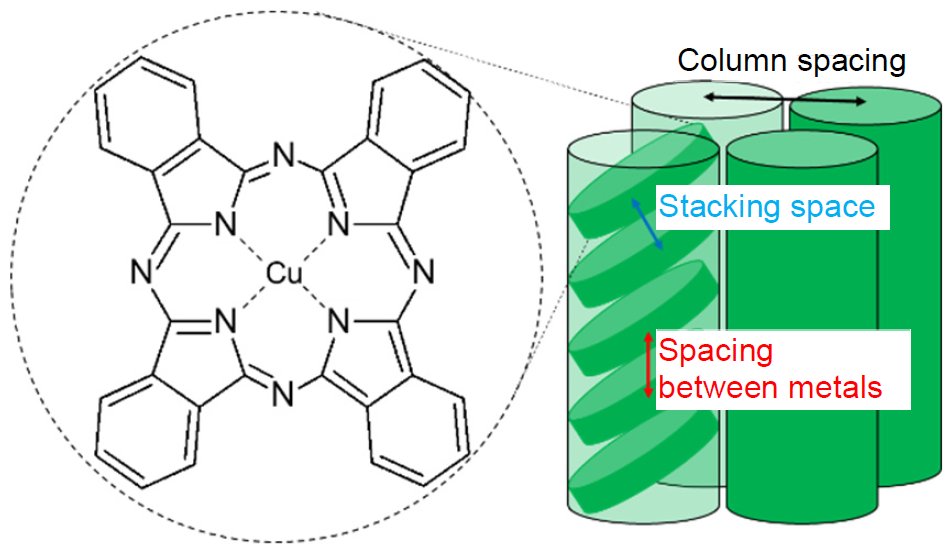
Figure 1: The molecular structure and the molecular packing structure of CuPc
Figure 2 shows the powder X-ray diffraction profile. We attempted indexing but did not succeed because the lattice constants could not be determined. Since the molecular stacking spacing in the molecular packing structure shown in Figure 1 is shorter than the column spacing, the peaks attributed to the molecular stacking structure are shifted to the high angle side in 2θ. Thus, the central metal distance is believed to be longer than 3.75 Å because a high-intensity peak, which probably comes from the molecular stacking structure, was detected at 2θ = 23.7° (3.75 Å).
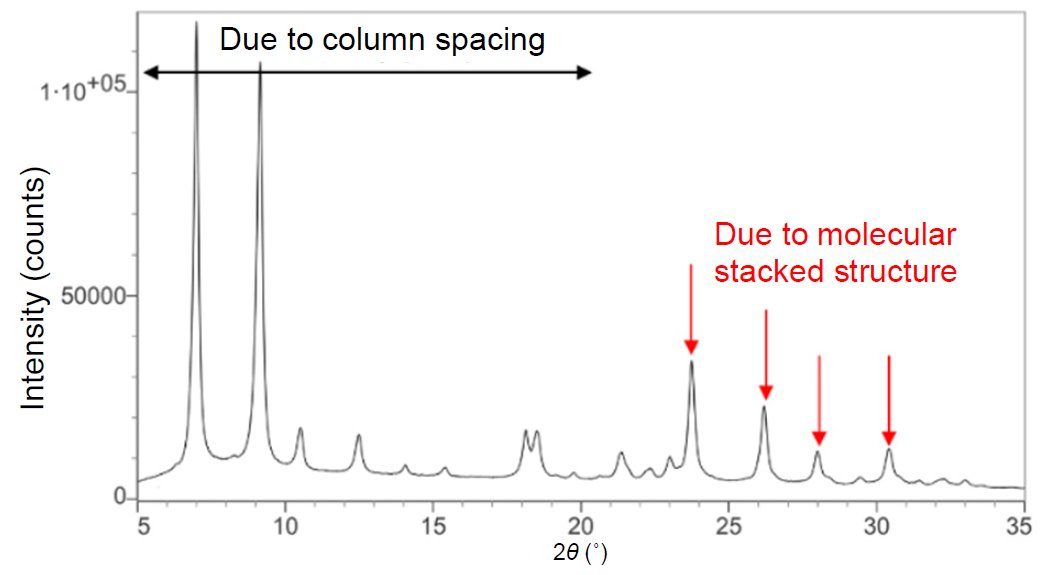
Figure 2: Powder X-ray diffraction profile measured using CuKα rays
Figure 3 shows the measured PDF pattern and a calculated PDF pattern from a single molecule of CuPc using the analysis software PDFgui(2). The comparison shows that the peak intensities equivalent to interatomic distances of 3.54 Å, 4.60 Å and 4.88 Å differ. The difference may occur because the intermolecular spacing is not taken into account in the single-molecule simulation. Taking into account the powder X-ray diffraction results, the central metal distance is suggested to be 4.60 Å or 4.88 Å.
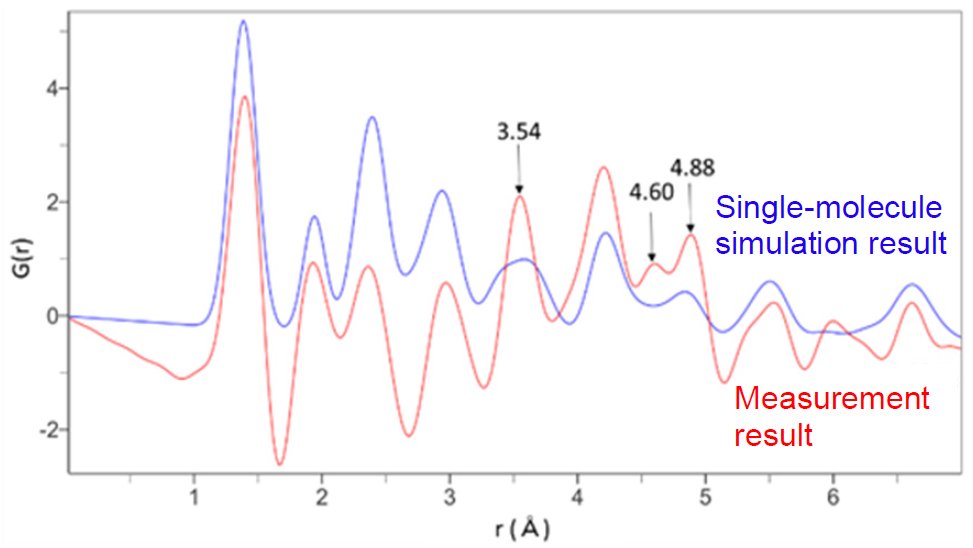
Figure 3: PDF pattern of the measurement result and the PDF pattern simulated with a single molecule
According to the crystal structure listed in the database (ICDD PDF-4/Organics), the central metal distance is 4.81 Å, which corresponds to the analysis result of 4.88 Å. These results suggest that PDF analysis enables calculating the central metal spacing, which is difficult to estimate by powder X-ray analysis.
References
(1) M. Yoshimoto and Y. Shiramata: Rigaku Journal (English version), 36(1) (2020) 10-18.
(2) P. Juhas, C. L. Farrow, X. Yang, K.R. Knox and S. J. L. Billinge: Acta Crystallogr., A71 (2015) 562-568.

Contact Us
Whether you're interested in getting a quote, want a demo, need technical support, or simply have a question, we're here to help.
