Evaluation of Crystallite Size and Pore Size distribution of Fuel Cell Materials
Introduction
A fuel cell is made of a membranelelectrode assembly (combination of anode, polymer electrolyte membrane, and cathode) connected to multiple cells consisting of gaskets and separators. Hydrogen and methanol gases supplied at the anode are decomposed into protons and electrons by catalytic reactions. In this reaction, platinum nanoparticles supported on carbon are used as the catalyst. Note that an increase in the average pore size of platinum catalysts is strongly involved with the degradation of fuel cells. Here we used an infrared heating high-temperature attachment and performed an in-situ measurement in a hydrogen gas atmosphere to evaluate the crystallite size of platinum nanoparticles. In addition, we evaluated the average pore size and the pore size distribution by performing a SAXS measurement on the platinum nanoparticles before and after supplying hydrogen gas.
Measurements and results
Figure 1 displays the changes in X-ray diffraction profiles of platinum nanoparticles (pore size of 2 nm to 3 nm, concentration approx. 40 mass%) while hydrogen gas was supplied to the Reactor X infrared heating high-temperature attachment. Figure 2 shows the SAXS profiles before and after supplying hydrogen gas. We calculated the crystallite size deriving from hydrogen gas supply based on the FWHM of the diffraction profile and found that it increased from 2.2 nm to 3.1 nm. On the other hand, in the SAXS profiles we found that the average pore size changed from 2.8 nm to 3.1 nm before and after supplying hydrogen gas. From these results, it can be assumed that the increases in crystallite size and average pore size were caused by the reduction reaction of the oxide film (which covered the platinum nanoparticles before supplying hydrogen gas) to metallic platinum. In conclusion, the infrared heating high-temperature attachment is useful for the in-situ measurement (to observe changes in diffraction profiles deriving from gas introduction) and the evaluation of the change in crystallite size of 1 nm or less. By combining the SAXS measurement, integrated analysis with the average pore size and the crystallite size is available as well.
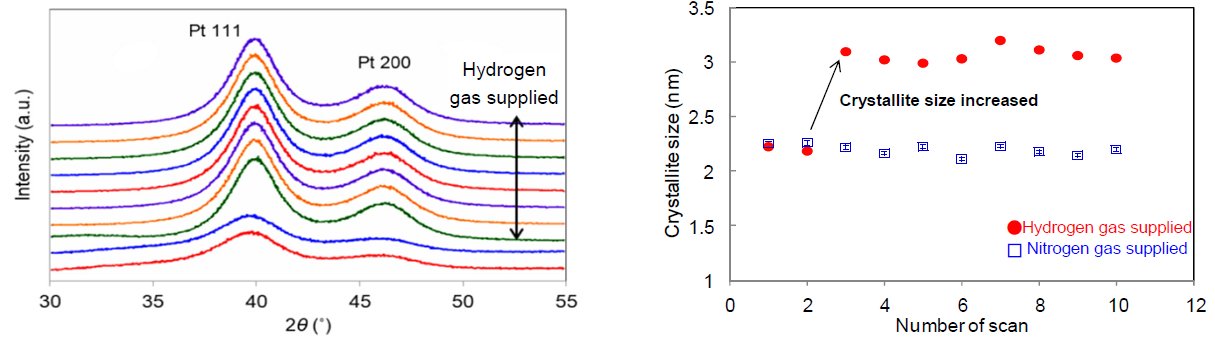 Figure 1: (Left) Change in X-ray diffraction profiles by hydrogen gas supply (Right) Change in crystallite size of platinum nanoparticle
Figure 1: (Left) Change in X-ray diffraction profiles by hydrogen gas supply (Right) Change in crystallite size of platinum nanoparticle
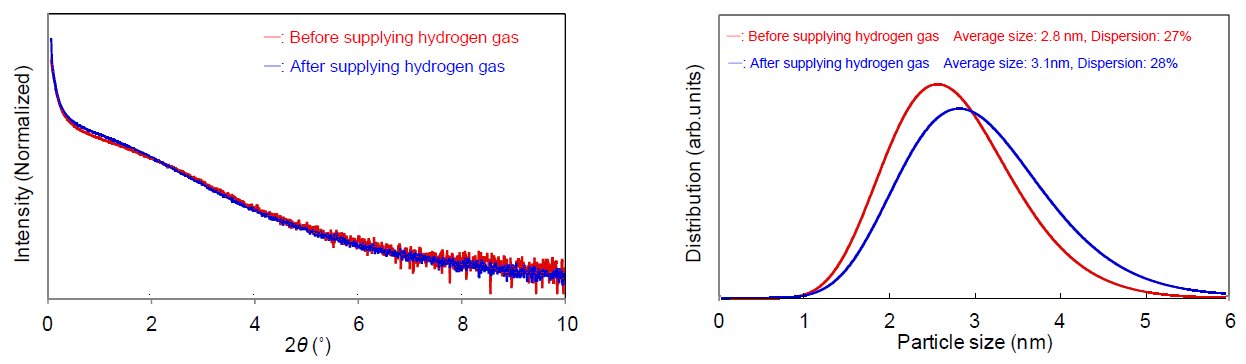
Figure 2: (Left) SAXS profiles before and after supplying hydrogen gas (Right) Pore size distribution analysis results of platinum nanoparticle
References
H.Yashiro: 17th Fall Meeting The Ceramic Soc. Jpn., (2004), 2P60.

Contact Us
Whether you're interested in getting a quote, want a demo, need technical support, or simply have a question, we're here to help.
