Crystal Phase Identification of Pharmaceutical Materials in the Freeze Drying Process
Introduction
Pharmaceuticals that rapidly dissolve in aqueous solutions (use-time dissolution type preparation) are manufactured by freeze drying. Freeze drying includes three steps: 1) “freezing” to freeze water and concentrating solutions, 2) “primary drying” to sublimate ice, and 3) “secondary drying” to evaporate water from solid parts. A high primary drying temperature can reduce drying time but, if this temperature is too high, structural collapse will occur. Thus, the maximum allowed temperature and proper annealing conditions that do not cause structural collapse have been examined. However, the conditions for freeze drying are not easily determined because the crystallization and transition behavior change depending on the type and concentration of additives. In this example, we performed in-situ observation of the crystalline phase transition during the actual freeze drying process using the low and middle sample temperature attachment.
Example of measurement and analysis
Mannitol is a sugar alcohol widely used as a diluent for use-time dissolution type preparation. Freeze drying simulation was performed using a 15-mass% aqueous solution of mannitol. Figure 1 shows the X-ray diffraction profiles of mannitol measured under conditions equivalent to the actual freezing process: -40°C (atmospheric pressure), the primary drying process: -20°C (reduced pressure), and the secondary drying process: 20°C and 40°C (reduced pressure for both). Using the common powder X-ray diffraction patterns registered in a database, the crystal forms at each temperature were identified, and mannitol was precipitated as a hemihydrate and β form during the freezing process. We also observed that these two phases existed up to 20°C during the secondary drying. After that, when the secondary drying temperature reached 40°C, it was found that the hemihydrate was dehydrated and transitioned to the α and δ types. This example indicates that using the low and middle temperature attachment enables the determination of the crystal form under various temperatures and degrees of vacuum. 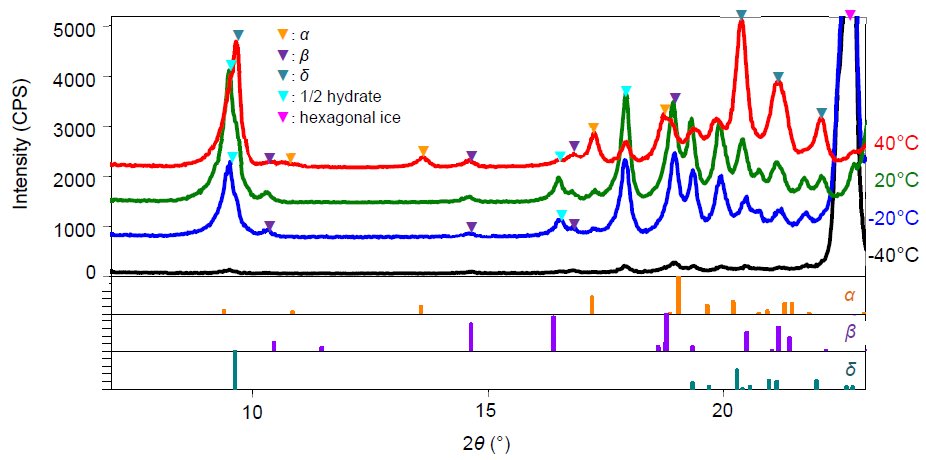 Figure 1: X-ray diffraction patterns of the 15 mass% mannitol aqueous solution measured at -40°C (atmospheric pressure), -20°C, 20°C, and 40°C (reduced pressure for all)
Figure 1: X-ray diffraction patterns of the 15 mass% mannitol aqueous solution measured at -40°C (atmospheric pressure), -20°C, 20°C, and 40°C (reduced pressure for all)

Contact Us
Whether you're interested in getting a quote, want a demo, need technical support, or simply have a question, we're here to help.
