Operando Measurement of Laminated Lithium-ion Battery using 2DD
Introduction
To develop lithium ion secondary batteries with high capacity, high reliability, and long life, it is essential to evaluate the stability of the electrode materials during the charge/discharge process. The laminate cell attachment enables the reproduction of a high-speed charge/discharge process while keeping the sample temperature constant, and simultaneously allows the collection of transmission X-ray diffraction images. Using this attachment with an X-ray diffractometer equipped with a Mo X‑ray source and a 2D detector collecting up to 131 diffraction images per second, it is possible to observe the rapid phase transition occurring inside the battery cell.
Measurement and result
Figure 1 shows a photo of the laminate cell attachment and the 2D detector. LiFePO₄ was selected as the positive electrode. Measurement using an exposure time of 10 sec was repeated while charging/discharging the laminate cell at 2C/1C (Here, 1C denotes the current based on ampere-hour rating for total charge / discharge in 1 hour, e.g. the charge at 2C completes in 0.5 hours and discharge at 0.5C does in 2 hours. ) Figure 2 shows a 2D diffraction image observed during the charging process. Diffraction signals characteristic to FePO₄ and LiFePO₄ were observed in the region indicated by the dotted line. Figure 3 shows the profile map of the region marked in Figure 2, the voltage graph and the diffraction peak intensities of FePO₄ and LiFePO₄. It was confirmed that the electrode material returned to the same crystal phase as before the measurement started.
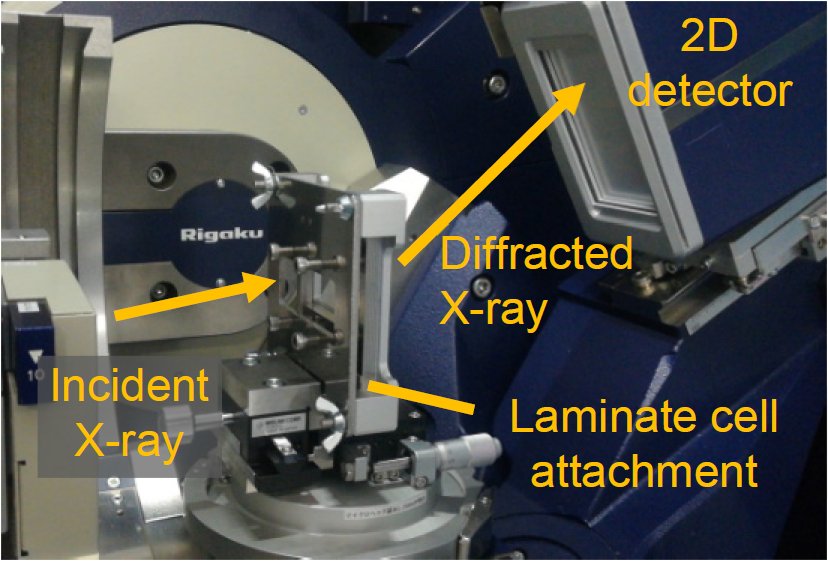
Figure 1: Laminate cell attachment.
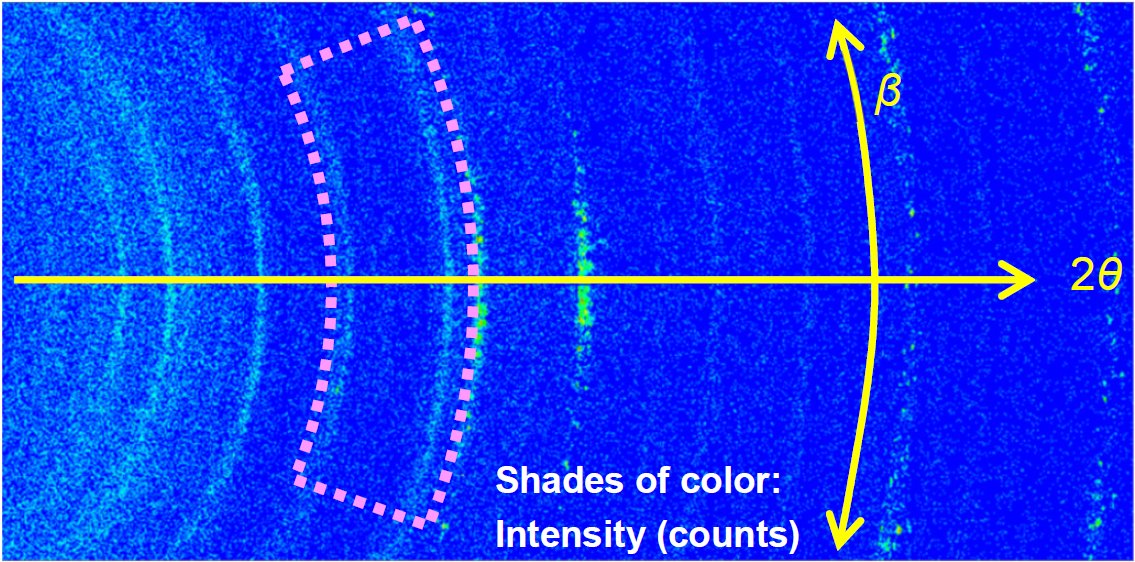
Figure 2: A 2D diffraction image observed in the charging process.
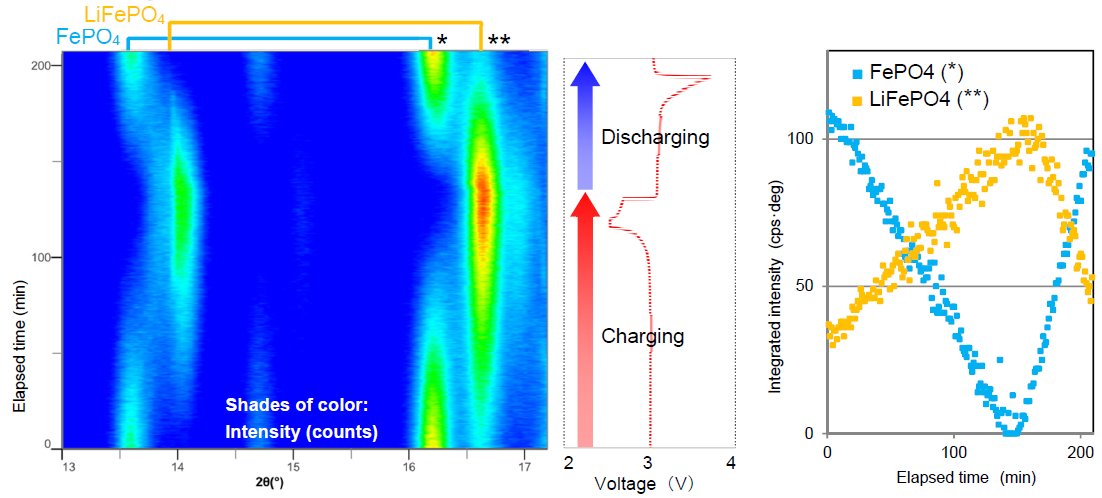
Figure 3: The profile map, voltage graph and diffraction peak intensity of FePO₄ and LiFePO₄.

Contact Us
Whether you're interested in getting a quote, want a demo, need technical support, or simply have a question, we're here to help.
