Background
Iron rust (ferric oxides) has two forms. One is hematite (Fe₂O₃), called red rust, and the other is magnetite (Fe₃O₄), called black rust. Ferric oxides exist abundantly in nature; since they have low impact on the human body and ecosystems, and they are cheap, they are used for various applications, such as a corrosion inhibitor, an abrasive agent, a colorant and a catalyst. These ferric oxides have different crystal structures, so they show different X-ray diffraction patterns. Using XRD, one can easily distinguish between these two oxides, and can also perform a quantitative analysis when the sample is a mixture of the two.
Investigation
Using Rigaku's SmartLab multipurpose diffractometer equipped with a D/teX one-dimensional X-ray detector, even when the source is a 2 kW sealed tube generator, one can perform the identification and quantification of the material in a short time.
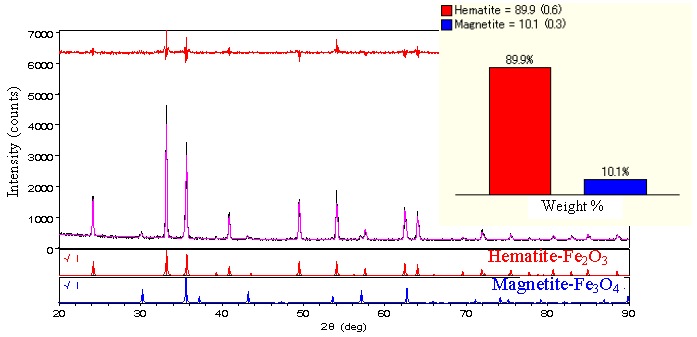
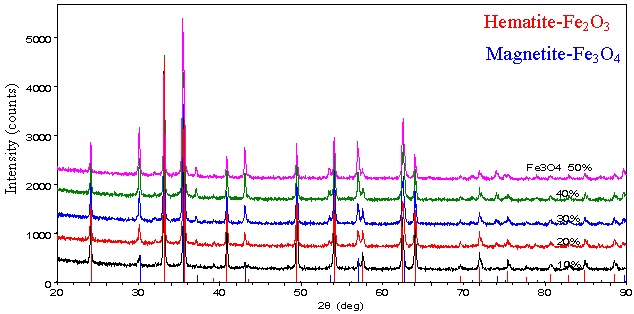
Samples blended 10, 20, 30, 40 and 50% of Fe3O4 into Fe2O3 were measured (Fig. 1). Even with data acquisition times as short as 3.5 minutes per measurement, sufficient intensities are obtained. Quantitative analysis results by the Rietveld method show good agreement with the known composition values (Fig. 2).
Optical system: focusing method
X-ray source: Cu Kα (40 kV/50 mA)
Measurement time: 3.5 minutes