Background
Zeolites, both naturally occurring and synthetic are currently used for catalysts, absorbents, and as molecular sieves. In the past 60 years, these aluminosilicate based compounds have proved highly versatile to both industry and research in areas of environmental clean-up, petroleum cracking, filtering/separations, and cation exchange materials.
Investigation
A frame work of 5 silicon and aluminum tetrahedra (tectosilicates), results in a cage-like structure with large open spaces. Depending on the range of d-spacing and other atoms present, the arrangement can be adjusted to yield channels or pores between 3 to 10 Å in size. Slight changes in the synthesis process can change the pores size or charge to select for a specific types of materials.
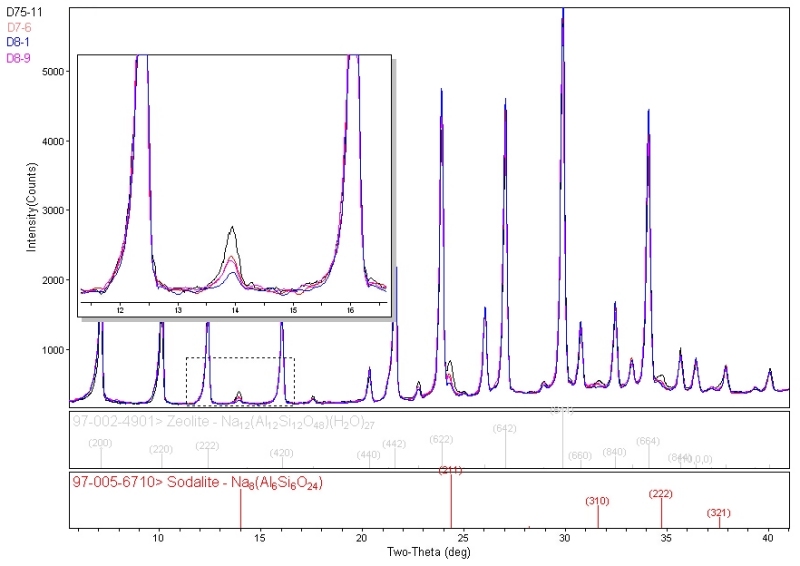
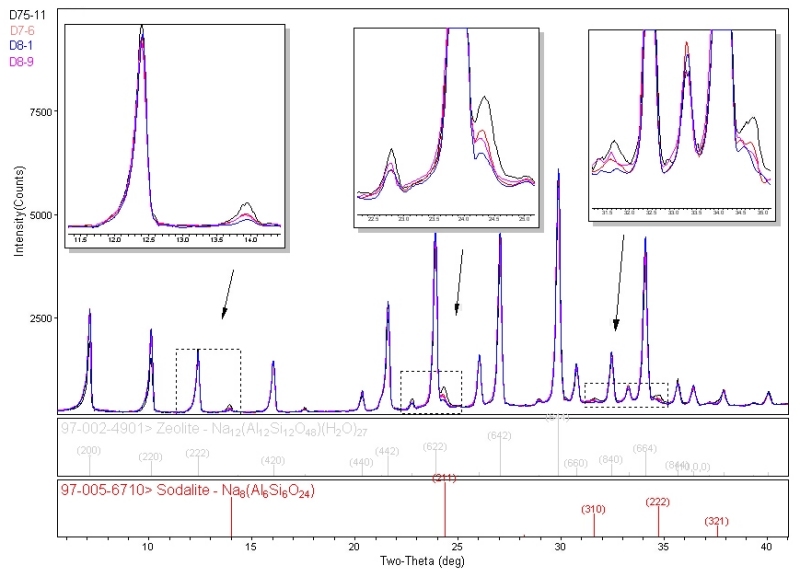
Individual compounds or impurities (phases) can be identified from the MiniFlex diffraction patterns by comparison with known databases published by ICDD( International Centre for Diffraction Data) and ICSD (Inorganic Crystal Structure Database). Over 1229 zeolite structures are currently available in the combined databases for identification of finished materials.